13.3
Impact Factor
Theranostics 2018; 8(8):2245-2248. doi:10.7150/thno.24181 This issue Cite
Editorial
Focused ultrasound with microbubbles induces sterile inflammatory response proportional to the blood brain barrier opening: Attention to experimental conditions
1. Frank Laboratory, Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Bethesda, MD 20892
2. National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health, Bethesda, MD 20892
Received 2018-2-9; Accepted 2018-2-15; Published 2018-3-8
Commentary-article in Theranostics, Volume 7, 3989
Abstract
This editorial highlights the findings of McMahon [1] and demonstrates the need for careful attention to experimental conditions that influence microbubble concentration and pharmacokinetics contributed to focused ultrasound-induced blood brain barrier opening and sterile inflammation.
The blood-brain barrier (BBB) maintains homeostasis, preventing the passage of toxins and cells into the brain that could induce inflammation and damage the surrounding elements of the neurovascular unit (NVU). Focused ultrasound (FUS) coupled with intravenous infusion of microbubbles (MB) can open the blood brain barrier and allow leakage of neurotherapeutics and plasma proteins into the parenchyma that can activate astrocytes and microglia [2]. Pulsed focused ultrasound (pFUS) induces stable MB oscillations, which radiate pressure waves on and through the NVU. Either stable oscillations, or possibly intertial caviation, could alter the parenchymal microenvironment to increase expression of the cytokines, chemokines, trophic factors (CCTF), and cell adhesion molecules (CAM) observed in a sterile inflammatory response (SIR) [2]. Transient SIR can both cause damage and stimulate repair mechanisms in the parenchyma [3, 4]. pFUS+MB increases interferon-γ expression within the parenchyma by stimulating a local immune response within the targeted parenchyma [2] and this cytokine can stimulate an innate immune response aiding in the clearance of amyloid plaques in Alzheimer's disease (AD) models [5, 6].
McMahon [1] reported that pFUS+MB-induced BBB disruption (BBBD) increased mRNA associated with inflammatory pathways involving nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) in brain obtained at 6 h post sonication, and that the SIR magnitude depended on the MB dose. Coupling pFUS with the US contrast agent Definity® at 10 µL/kg did not significantly elevate transcriptomic constituents of NFκB pathways, resulting in significantly less (p<0.01) signal intensity (SI) changes on gadolinium (Gd)-T1 weighted (w) images when compared to pFUS+Definity® at 100 µL/kg when the sonication peak negative pressure (PNP) was controlled by passive cavitation detection (PCD). pFUS+Definity® at 100 µL/kg resulted in a SIR evidenced by increasing mRNA expression of pro-inflammatory factors, larger SI changes that were homogenous in appearance, and the appearance of hypointense voxels on T2*w imaging 4 h post-sonication that were consistent with parenchymal damage. pFUS+Definity® at 100 µL/kg using PCD feedback did result in an inflammatory response with transcriptomic elevations in Ccl5, Faslg, Tnf, Il1b and Icam1 presumably in the absence of inertial cavitation. A linear correlation was observed between transcriptomic responses to pFUS+MB-induced BBBD and SI changes on MRI. However, the changes in SI and mRNA expression following pFUS+Definity® resulted in correlations with R2 ranging from 0.693 to 0.386 (depending on the CCTF), implying that between approximately 31% to 62% of the mRNA expression variability is unrelated to SI changes. The mRNA encoding for a range of IL1b, IL1a, and TNF associated with SIR had <42% of their variability accounted for based on SI alone, leaving other factors contributing to changes in SI unaccounted for [1]. A lack of transcriptomic changes does not necessarily indicate unaltered protein expression since cells contain baseline quantities of mRNA available for protein translation without transcribing new mRNA. It is also possible that transcriptomic responses consistent with activated NFκB pathways to pFUS+Definity® at 10 µL/kg would have been observed if tissue sampling had been performed at other time points [2].
These observations underscore the vital need to address questions regarding pFUS+MB-induced BBBD relating to MRI SI changes: 1) What magnitude of SI changes on Gd-T1w MRI precisely define BBBD? 2) How does the magnitude of SI changes following pFUS+MB predict adequate parenchymal delivery of neurotherapeutics or stimulation of molecular and or immune responses?
McMahon attempted to replicate parts of a previous study by Kovacs [2], which used a pFUS peak negative pressure of 0.3 MPa (in water) at a transducer frequency of 589 kHz (translating to a mechanical index of 0.39)—below the reported limits of BBBD with erythrocyte extravasation [7] that induced a SIR in the area of BBBD. Important differences between the two studies lead to some overstating of the data in [1]. McMahon attributes the SIR in Kovacs [2] to the fact that the OptisonTM MB dose (500 µL/kg) was 10-fold higher than the clinical dose and thus comparable to Definity® at 100 µL/kg (10-fold higher than its clinical US imaging dose). This relationship may not hold true for pFUS+MB induced BBBD. The two MB preparations are different in size, dispersity, and concentration (Definity®=1.2×1010 MB/mL, OptisonTM=5-8×108 MB/mL), which may affect their ability to open the BBB. Differences in total numbers of MB infused, infusion rates (IR), oxygenation states, and number of targets influence BBBD may alter the downstream parenchymal molecular responses. Kovacs infused OptisonTM (5-8×107 MB) at 100 µL/min over 1 min starting 30 s before sonication in rats inhaling 100% O2 that resulted in homogeneous BBBD over 9 focal spots based on Gd-T1w images [2]. McMahon [1] infused Definity® diluted in saline at 10 or 100 µL/kg (1.2×108 MB/kg or 1.2×109 MB/kg, respectively) at 120 µL/min in rats on medical air (21% O2) sonicating at various PNP in one focal area. The intravascular half-life (T1/2) of both MBs is 1.3 min in air and 0.72 min on 100% O2 [8-10]. MBs are eliminated through the lungs and plasma concentration depends on the initial dose, IR, T1/2, and oxygenation status.
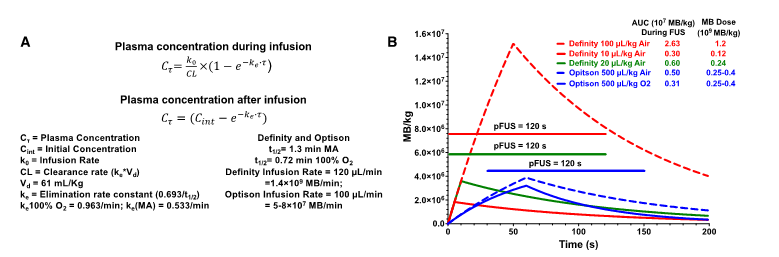
Figure 1 contains the equations and graphs from pharmacokinetic modeling (http://www.gatewaycoalition.org/files/hidden/deliv/ch3/3_5f.htm) of Definity® at 100 µL/kg, 20 µL/kg, and 10 µL/kg (air), IR=120 µL/min and OptisonTM at 500 µL/kg on air or 100% O2, IR=100 µL/min, all with 120 s of pFUS [1, 2]. The Definity® dose of 20 µL/kg is based on a previous report [11]. During the total FUS “on” time, the area under the curve (AUC) was 2.63×107 MB/kg for Definity® at 100 µL/kg on air. This was >8.4× greater than OptisonTM at 0.25-0.4×109 MB/kg (500 µL/kg) while inhaling 100% O2. Kovacs' MB dosing and pFUS protocol resulted in essentially no difference in AUC as Definity® at 10 µL/kg, underscoring the importance of IR and inhaled O2 [2]. The AUC calculations assumed fixed FUS pressures because the data presented by McMahon [1] do not allow determination of at which US burst number (i.e., time after initiating pFUS) stable cavitation would be detected. However, we can determine that AUC differences between constant or ramped pressures are negligible. McMahon [1] states that PNP was initially set at 0.128 MPa and ramped up to a mean of 0.192 MPa (0.064 MPa difference). Increasing by 0.008 MPa/cycle means that 0.192 MPa would be achieved by the 9th pulse. If we generously assume that no cavitation whatsoever occured during the first 8 pulses and remove those data points from the integration, the AUC values only change by <1% (2.63 vs. 2.61 for 100 µL/kg; 0.300 vs. 0.298 for 10 µL/kg). Of course, in reality, stable cavitation likely begins before the transducer reaches an inertial cavitation threshold. The modeling demonstrates that approaches to MB dosing demand at least quantifying circulating MB as discrete cavitation nuclei. It is clearly insufficient to simply adjust volumes of solution when dealing with different formulations. MB dosing by gas volume/kg correlates with BBBD and represents an alternative to liquid-volume-based dosing. MB size affects physical interactions with pFUS, making certain diameters better BBBD agents at a given frequency [12]. Moreover, vascular heterogeneity will give different vessel types varying susceptibility to BBBD for disperse MB sizes.
We agree with McMahon's findings that SIR will be proportional to MB dose on some level, but the picture is extremely complex. For pFUS+MB to emerge as a viable therapeutic option will require: 1) quantitative imaging standards that define adequate BBBD (i.e., MRI parameters and findings); 2) validated therapeutic and molecular/immunogical outcomes; and 3) the understanding of how the magnitude of BBBD is influenced by MB number, size, dispersity, IR and clearance. There are wide ranges of MRI parameters that can be manipulated to increase the conspicuity of BBBD following sonication, which also need standardization. Defining the infused MB concentration, IR and appropriate pFUS parameters used when canvassing brain pathologies, while investigating safety and the specific value of pFUS+MB to induce a SIR are necessary in stimulating an immune response.
There are several reports that pFUS+Definity® at higher doses (20-80 µL/kg) was needed to open the BBB and clear amyloid plaques in mouse AD models [13-15]. In the canine model of aging [16], pFUS with PCD feedback was coupled with an infusion of Definity® at 20 µL/kg. Although BBB opening was detected on Gd-T1w MRI, the clearance of amyloid plaques did not reach significance for the entire cohort possibly due to the small sample size. An SIR following pFUS+MB BBBD would be likely to occur with a Definity® dose ≥20 µL/kg compared to Definity® at 10 µL/kg used by McMahon [1]. Indeed, an SIR that includes elevation in CCTF and CAM could be important to initiate an immune response that would help clear amyloid plaques [5, 6]. If the minimal SIR profile observed by McMahon at 10 µL/kg [1] was induced in the transgenic AD mouse, there is a high possibility that little changes to pathology would be observed. Noninvasive image guided pFUS+MB opening of the BBB does hold potential compared to other invasive techniques [17], but still requires standardized parameters for US, MB dosing, IR, and MRI protocols. This leaves subsequent studies that investigate biology and therapeutic effectiveness sometimes difficult to interpret and underscores the importance of using acoustic emissions to calibrate PNP, which widens the safety window of pFUS+MB and improves consistency in BBBD.
(A) Pharmacokinetic equations and definition of parameters used for modeling clearance of MB. (B) Graphs of models using either Definity® or OptisonTM at various MB/kg doses and IR. Line represents on-time of pFUS.
Acknowledgements
This work was supported by the Intramural Research Programs of the Clinical Center and the National Institute of Biomedical Imaging and Bioengineering at the National Institutes of Health.
References
1. McMahon D, Hynynen K. Acute Inflammatory Response Following Increased Blood-Brain Barrier Permeability Induced by Focused Ultrasound is Dependent on Microbubble Dose. Theranostics. 2017;7:3989-4000
2. Kovacs ZI, Kim S, Jikaria N, Qureshi F, Milo B, Lewis BK. et al. Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation. Proc Natl Acad Sci U S A. 2017;114:E75-E84
3. Amantea D, Micieli G, Tassorelli C, Cuartero MI, Ballesteros I, Certo M. et al. Rational modulation of the innate immune system for neuroprotection in ischemic stroke. Front Neurosci. 2015;9:147
4. Gadani SP, Walsh JT, Lukens JR, Kipnis J. Dealing with Danger in the CNS: The Response of the Immune System to Injury. Neuron. 2015;87:47-62
5. Baruch K, Deczkowska A, Rosenzweig N, Tsitsou-Kampeli A, Sharif AM, Matcovitch-Natan O. et al. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer's disease. Nat Med. 2016;22:135-7
6. Schwartz M, Deczkowska A. Neurological Disease as a Failure of Brain-Immune Crosstalk: The Multiple Faces of Neuroinflammation. Trends Immunol. 2016;37:668-79
7. Chu PC, Chai WY, Tsai CH, Kang ST, Yeh CK, Liu HL. Focused Ultrasound-Induced Blood-Brain Barrier Opening: Association with Mechanical Index and Cavitation Index Analyzed by Dynamic Contrast-Enhanced Magnetic-Resonance Imaging. Sci Rep. 2016;6:33264
8. Itani M, Mattrey RF. The effect of inhaled gases on ultrasound contrast agent longevity in vivo. Mol Imaging Biol. 2012;14:40-6
9. McDannold N, Zhang Y, Vykhodtseva N. The Effects of Oxygen on Ultrasound-Induced Blood-Brain Barrier Disruption in Mice. Ultrasound Med Biol. 2017;43:469-75
10. Mullin L, Gessner R, Kwan J, Kaya M, Borden MA, Dayton PA. Effect of anesthesia carrier gas on in vivo circulation times of ultrasound microbubble contrast agents in rats. Contrast Media Mol Imaging. 2011;6:126-31
11. McMahon D, Bendayan R, Hynynen K. Acute effects of focused ultrasound-induced increases in blood-brain barrier permeability on rat microvascular transcriptome. Sci Rep. 2017;7:45657
12. Choi JJ, Feshitan JA, Baseri B, Wang S, Tung YS, Borden MA. et al. Microbubble-size dependence of focused ultrasound-induced blood-brain barrier opening in mice in vivo. IEEE Trans Biomed Eng. 2010;57:145-54
13. Burgess A, Dubey S, Yeung S, Hough O, Eterman N, Aubert I. et al. Alzheimer disease in a mouse model: MR imaging-guided focused ultrasound targeted to the hippocampus opens the blood-brain barrier and improves pathologic abnormalities and behavior. Radiology. 2014;273:736-45
14. Liu M, Jevtic S, Markham-Coultes K, Ellens NPK, O'Reilly MA, Hynynen K. et al. Investigating the efficacy of a combination Abeta-targeted treatment in a mouse model of Alzheimer's disease. Brain Res. 2018;1678:138-45
15. Jordao JF, Thevenot E, Markham-Coultes K, Scarcelli T, Weng YQ, Xhima K. et al. Amyloid-beta plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp Neurol. 2013;248:16-29
16. O'Reilly MA, Jones RM, Barrett E, Schwab A, Head E, Hynynen K. Investigation of the Safety of Focused Ultrasound-Induced Blood-Brain Barrier Opening in a Natural Canine Model of Aging. Theranostics. 2017;7:3573-84
17. Neuwelt E, Abbott NJ, Abrey L, Banks WA, Blakley B, Davis T, Engelhardt B, Grammas P, Nedergaard M, Nutt J, Pardridge W, Rosenberg GA, Smith Q, Drewes LR. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008Jan;7(1):84-96 Review
Author contact
![]() Corresponding author: jfrankgov
Corresponding author: jfrankgov
 Global reach, higher impact
Global reach, higher impact