13.3
Impact Factor
Theranostics 2018; 8(14):3797-3807. doi:10.7150/thno.24941 This issue Cite
Research Paper
Development of Paper-Based Analytical Devices for Minimizing the Viscosity Effect in Human Saliva
1. Ph.D program in Clinical Biochemistry and Molecular Medicine, Department of Clinical Chemistry, Faculty of Allied Health Sciences, Chulalongkorn University, Bangkok 10330, Thailand.
2. Department of Chemistry, Colorado State University, Fort Collins, Colorado 80523, United States.
3. The School of Biomedical Sciences, Institute of Health and Biomedical Sciences, Queensland University of Technology, Brisbane, Queensland, 4059, Australia.
4. Department of Clinical Chemistry, Faculty of Allied Health Sciences, Chulalongkorn University, Bangkok, 10330, Thailand.
5. Electrochemistry and Optical Spectroscopy Center of Excellence, Chulalongkorn University, Bangkok, 10330, Thailand.
6. Department of Chemical and Biological Engineering, Colorado State University, Fort Collins, Colorado 80523, United States.
Abstract
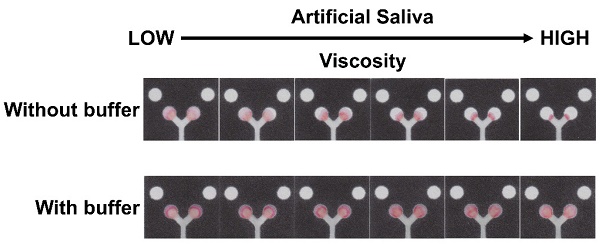
Rationale: Saliva as a sample matrix is rapidly gaining interest for disease diagnosis and point-of-care assays because it is easy to collect (non-invasive) and contains many health-related biomarkers. However, saliva poses particular problems relative to more common urine and blood matrices, which includes low analyte concentrations, lack of understanding of biomolecule transportation and inherent viscosity variability in human samples. While several studies have sought to improve assay sensitivity, few have addressed sample viscosity specifically. The goal of this study is to minimize the effect of sample viscosity on paper-based analytical devices (PADs) for the measurement of pH and nitrite in human saliva.
Methods: PADs were used to measure salivary pH from 5.0 to 10.0 with a universal indicator consisting of chlorophenol red, phenol red and phenolphthalein. Nitrite determination was performed using the Griess reaction. Artificial saliva with viscosity values between 1.54 and 5.10 mPa∙s was tested on the proposed PAD. To ensure the proposed PADs can be tailored for use in-field analysis, the devices were shipped to Australia and tested with human specimens.
Results: Initial experiments showed that viscosity had a significant impact on the calibration curve for nitrite; however, a more consistent curve could be generated when buffer was added after the sample, irrespective of sample viscosity. The linear range for nitrite detection was 0.1 to 2.4 mg/dL using the improved method. The nitrite measurement in artificial saliva also showed a good correlation with the standard spectrophotometry method (p=0.8484, paired sample t-test, n=20). Measured pH values from samples with varying viscosities correlated well with the results from our pH meter.
Conclusions: The inherent variation of salivary viscosity that impacts nitrite and pH results can be addressed using a simple washing step on the PAD without the need for complex procedures.
Keywords: Paper-based analytical devices, saliva analysis, pH testing, nitrite, viscosity, oral cancer biomarkers
 Global reach, higher impact
Global reach, higher impact