13.3
Impact Factor
Theranostics 2018; 8(14):3949-3963. doi:10.7150/thno.26161 This issue Cite
Research Paper
Polylactide-tethered prodrugs in polymeric nanoparticles as reliable nanomedicines for the efficient eradication of patient-derived hepatocellular carcinoma
1. The First Affiliated Hospital; Key Laboratory of Combined Multi-Organ Transplantation, Ministry of Public Health; Key Laboratory of Organ Transplantation of Zhejiang Province, School of Medicine; Zhejiang University, Hangzhou 310003, P. R. China.
2. Shenzhen Key Laboratory of Hepatobiliary Disease, Shenzhen Third People's Hospital, Shenzhen 518112, P. R. China
3. Center for Bionanoengineering and State Key Laboratory of Chemical Engineering, Department of Chemical and Biological Engineering, Zhejiang University, Hangzhou, 310027, P. R. China.
Abstract
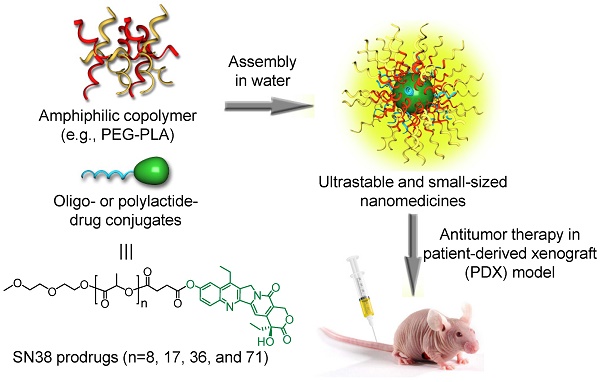
Nanomedicines have been extensively explored for cancer treatment, and their efficacies have arguably been proven in various cancer cell-derived xenograft (CDX) mouse models. However, they generally fail to show such therapeutic advantages in patients because of the huge pathological differences between human tumors and CDX models.
Methods: In this study, we fabricated colloidal ultrastable nanomedicines from polymeric prodrugs and compared the therapeutic efficacies in hepatocellular carcinoma (HCC) CDX and clinically relevant patient-derived xenograft (PDX) mouse models, which closely mimic human tumor pathological properties. Working towards this goal, we esterified a highly potent SN38 (7-ethyl-10-hydroxycamptothecin) agent using oligo- or polylactide (oLA or PLA) segments with varying molecular weights.
Results: The resulting SN38 conjugates assembled with polyethylene glycol-block-polylactic acid to form systemically injectable nanomedicines. With increasing PLA chain length, the SN38 conjugates showed extended retention in the nanoparticles and superior antitumor activity, completely eradicating xenografted tumors in both mouse models. Our data implicate that these small-sized and ultrastable nanomedicines might also efficaciously treat cancer in patients. More interestingly, the systemically delivered nanomedicines notably alleviated the incidence of bloody diarrhea.
Conclusion: Our studies demonstrate that the appropriate molecular editing of anticancer drugs enables the generation of better tolerated cytotoxic nanotherapy for cancer, which represents a potentially useful scaffold for further clinical translation.
Keywords: cancer nanomedicine, polylactide, SN38 prodrug, self-assembly, patient-derived xenograft model
 Global reach, higher impact
Global reach, higher impact