13.3
Impact Factor
Theranostics 2018; 8(17):4710-4732. doi:10.7150/thno.26284 This issue Cite
Research Paper
Nanoparticles for diagnosis and therapy of atherosclerosis and myocardial infarction: evolution toward prospective theranostic approaches
1. Advanced Center for Chronic Diseases (ACCDiS), Facultad Ciencias Químicas y Farmaceuticas, Universidad de Chile, Santiago 8380492, Chile.
2. Departamento de Ciencias y Tecnología Farmacéuticas, Facultad de Ciencias Químicas y Farmacéuticas, Universidad de Chile, Santiago 8380494, Chile.
3. Pharmaceutical Biomaterial Research Group, Department of Health Sciences, Luleå University of Technology, Luleå 97187, Sweden
4. Departamento de Ciencias Quimicas, Facultad de Ciencias Exactas, Universidad Andres Bello, Republica 275, 8370146, Santiago, Chile.
5. Advanced Center for Chronic Diseases (ACCDiS), División de Enfermedades Cardiovasculares, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile.
6. Advanced Center for Chronic Diseases (ACCDiS), & Centro de Estudios en Ejercicio, Metabolismo y Cáncer (CEMC), Instituto de Ciencias Biomedicas (ICBM), Facultad de Medicina, Universidad de Chile, Santiago 8380492, Chile.
7. Department of Internal Medicine (Cardiology Division), University of Texas Southwestern Medical Center, Dallas, Texas, USA.
8. Departamento de Química Farmacológica y Toxicológica, Facultad de Ciencias Químicas y Farmacéuticas, Universidad de Chile.
Received 2018-3-25; Accepted 2018-6-29; Published 2018-9-9
Abstract
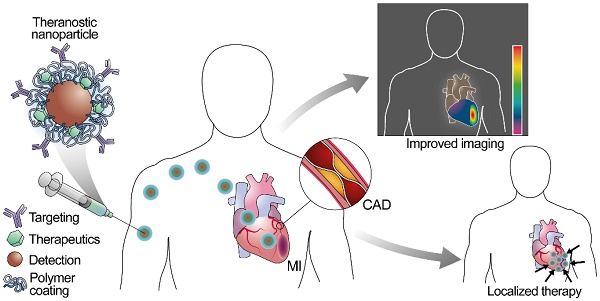
Cardiovascular diseases are the leading cause of death worldwide. Despite preventive efforts, early detection of atherosclerosis, the common pathophysiological mechanism underlying cardiovascular diseases remains elusive, and overt coronary artery disease or myocardial infarction is often the first clinical manifestation. Nanoparticles represent a novel strategy for prevention, diagnosis, and treatment of atherosclerosis, and new multifunctional nanoparticles with combined diagnostic and therapeutic capacities hold the promise for theranostic approaches to this disease. This review focuses on the development of nanosystems for therapy and diagnosis of subclinical atherosclerosis, coronary artery disease, and myocardial infarction and the evolution of nanosystems as theranostic tools. We also discuss the use of nanoparticles in noninvasive imaging, targeted drug delivery, photothermal therapies together with the challenges faced by nanosystems during clinical translation.
Keywords: Nanoparticles, theranostics, atherosclerosis, myocardial infarction, cardiovascular diseases.
Introduction
Cardiovascular diseases (CVD) are the most common cause of mortality in men and women worldwide [1]. In 2015, 17.7 million people died from CVD corresponding to 31% of all global deaths [1]. In Europe, more than 85 million people were living with a cardiovascular condition in 2015 [2], and in the US this number reached 92.1 million people from 2011 to 2014 [3]. Within CVD-related deaths, 45.1% are caused by coronary artery diseases (CAD), defined as a decrease in myocardial blood supply due to atherosclerotic plaque occlusion. Ruptures of atherosclerotic plaques are the cause of about 70% of myocardial infarction (MI) [4]. In the US, one person suffers an MI every 40 seconds [3]. CVD also represent a substantial economic burden for healthcare systems, with direct and indirect costs rising to an annual average of $316 billion in the US [3]. These alarming statistics are partially explained by an aging population, coupled with a modern lifestyle that increases the prevalence of cardiovascular risk factors such as high non-HDL cholesterol levels, hypertension, diabetes, obesity, tobacco use, lack of physical activity, and unhealthy diet [5].
Despite advances in diagnosis and treatment of CAD, early detection of atherosclerotic plaque remains elusive, and often MI is the first clinical manifestation of the atherosclerotic process [6]. Primary prevention strategies often rely on cardiovascular risk scores that predict the likelihood of CAD based on conventional factors such as age, sex, hypertension, diabetes, dyslipidemia, and smoking [5,7]. While these scores are widely used for population screening, they remain imprecise on an individual basis [6]. The application of non-invasive imaging techniques for evaluation of subclinical atherosclerotic burden represents an exciting alternative for early detection of individuals at high cardiovascular risk [8]. In subjects with suspected CAD, classical imaging approaches based on coronary stenosis evaluation are valuable in the symptomatic management of patients with angina. However, the roles of conventional imaging in predicting clinical outcomes remain controversial [9], since MI frequently evolves from regions of mild to moderate stenosis [10]. An ideal imaging method should report not only the volume of plaque within the coronary vessels but also adverse plaque characteristics such as large necrotic core, inflammation, intraplaque hemorrhage, thin fibrous cap, and microcalcifications [6].
In addition to the challenges in the diagnosis of atherosclerosis, new and better therapeutic alternatives are needed to reduce the residual risk of adverse cardiovascular effects in high-risk subjects even after standard therapy [11]. For subjects undergoing MI, while rapid restoration of coronary blood flow is the standard of care to minimize the infarct size [12], the restoration of blood flow itself causes irreversible myocardial damage in a process called myocardial reperfusion injury [13]. Despite advances in the studies on the molecular mechanisms involved in reperfusion-induced cell death, treatments focused on limiting the reperfusion damage (e.g., antioxidant therapy, calcium channel blockers, anti-inflammatory treatment, atorvastatin, adenosine) have produced disappointing results in the clinical setting [14]. The pursuit of new therapeutics for atherosclerosis and its consequences remains an open field of research.
Nanotechnology for diagnosis and therapy of cardiovascular diseases
Nanotechnology is a multidisciplinary research field involving design, synthesis, and characterization of materials with controlled shapes and sizes at the nanometer scale (10-9 meters) [15]. One of the most active research areas of nanotechnology is nano-biotechnology or nanomedicine, which is a cutting-edge field that involves the application of nanostructures in medicine and health care for the prevention, diagnosis, and treatment of diseases [16]. Typically, nanotechnology includes the understanding and handling of nanoparticles and nanostructures ranging from 1 to 100 nm, however, within the biomedical community, slightly larger structures also are regarded as nanoparticles [17]. At these dimensions, nanoparticles show unique physicochemical and biological properties (e.g., ability to cross the cell membrane and tissue barriers), thus allowing interaction with cell structures, which have similar sizes, such as proteins and other macromolecules inside cells [18]. These types of interactions occur at molecular levels with a high degree of specificity and reactivity [18]. Thus, the nanoparticles can stimulate, respond, and interact with target cells or tissues in controlled processes to produce desired physiological responses while minimizing undesirable effects. An example of a potential application is when nanoparticles target a specific injury site in the heart, and control the release of therapeutic molecules, which can be encapsulated, entrapped, conjugated, or adsorbed [16]. Other outstanding properties of some nanoparticles, such as surface plasmon resonance, high specific surface area and reactive area, magnetism, and morphological and structural changes by an externally applied stimulus, allow researchers to explore new alternatives to traditional diagnostic and therapeutic methods [19,20]. For instance, hybrid nanoparticles and nanocomposites generate multifunctional characteristics with great potential for more than one clinical purpose, such as theranostic applications [19-22].
Nanomedicines have been applied to treat and diagnose several diseases with promising results, but medical imaging and oncology are still the most active areas of development. Some examples of clinically approved intravenous nanoparticles are Doxil® (liposomal composition with doxorubicin for the treatment of various cancers) and Resovist® (iron carboxydextran colloid as magnetic resonance imaging (MRI) contrast agent for imaging of liver lesions) [23]. Nanoparticles that are currently in clinical trials are mainly aimed at the treatment of several cancers. These include mostly liposome-based formulations but also others based on polymers, micelles, and inorganic nanoparticles made primarily of gold, iron oxide, and silica [23].
The field of CVD poses significant clinical challenges that can be approached with nanomedicines. In this review, we will summarize the role of different nanostructures in the search for better diagnostic and therapeutic approaches to tackle the rising epidemic of CVD such as atherosclerosis and MI.
Historical review of nanoparticles used in diagnosis and therapy of atherosclerosis
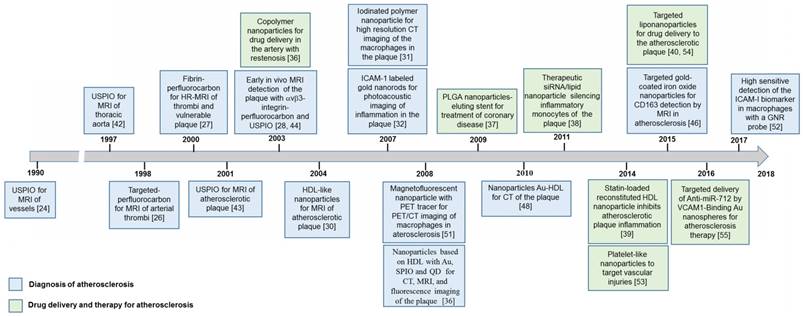
Figure 1 shows relevant studies on nanotechnology used in the treatment or diagnosis of atherosclerosis. This timeline scheme was made using the Web of Knowledge's database, selecting the publications with both greater number of citations and those highlighted with innovative nanosystems for these cardiovascular applications.
The application of nanoparticles in vascular diseases began with the use of iron oxide nanoparticles having magnetic properties as a vascular contrast agent for MRI in the early 90´s [24]. Superparamagnetic iron oxide nanoparticles (SPION) and small superparamagnetic iron oxide (USPIO) nanoparticles have attracted attention due to their considerably longer blood half-life and their outstanding performance as MRI contrast agents for the study of the atherosclerotic plaque [24]. During the 90´s, these superparamagnetic nanoparticles were coated with different polymers and biomolecules to improve their bioavailability and interaction with specific tissues [25]. Since the late 90´s and early 00´s, other nanoparticles used for MRI included lipid‑encapsulated perfluorocarbon emulsions with gadolinium and high-density lipoprotein (HDL)-like nanoparticles [26-30]. During these years, researchers also used targeting strategies to improve the specificity of the nanoparticles-based contrast agents [26-29]. In 2007, other types of nanoparticles based on polymers and gold were used as contrast agents for X-ray computed tomography (CT) imaging and photoacoustic imaging [31,32]. The applications of nanoparticles for atherosclerosis in the 90's and the first decade of the 00's were mostly focused on contrast agents to improve the visualization and analysis of conventional imaging techniques. Since 2005, several nanosystems with multifunctional properties have been developed for multimodal imaging such as positron-emission tomography (PET)-CT, PET-MRI, and MRI-fluorescence [33-35].
Nanosystems for treatment of vascular diseases, such as atherosclerosis, have also been developed since the 00's. These were mainly based on polymer nanoparticles used as vehicles for drugs [36,37]. In the last decade, lipid nanoparticles, such as liposomes and HDL-like nanoparticles, have also been used as vehicles for drug delivery due to their high biocompatibility [38, 39]. When used as hybrid nanosystems with nanoparticle-based contrast agents or fluorescent cues, these nanoparticles can also be used for multimodal imaging [34,40].
The above timeline in Figure 1 displayed the evolution of nanoparticles or nanosystems with multifunctional properties due to the combination of materials and biomolecules for the safe and effective diagnosis and treatment of atherosclerosis. More details about studies on these nanosystems and their specific applications are discussed below.
Nanoparticles for diagnosis of atherosclerosis
Nanomaterials have interesting properties that could be used to improve some of the techniques currently available for diagnosis. A chronological description of studies on nanoparticles used in MRI, CT, PET, photoacoustic imaging, multimodal imaging, and biomarkers detection applied to atherosclerosis is presented below.
Timeline of nanotechnology for therapy and diagnosis of atherosclerosis.
Magnetic resonance imaging
MRI combines high spatial and temporal resolution with a variety of readouts of plaque and vessel morphology. The first magnetic nanoparticles used for imaging of vessels were the superparamagnetic iron oxide nanoparticles (SPION) AMI-25 (Endorem; Laboratoire Guerbet, Aulnaysous-Bois, France), which were prepared as a stable aqueous colloidal suspension of magnetite (Fe3O4). The iron oxide was coated with a noncovalently bonded low-molecular-weight dextran for intravenous use. The diameter of the AMI-25 particles ranged from 120 to 180 nm, and they were biodegradable (i.e., the incorporation of iron content into hemoglobin) [41].
In the early 90´s, Weissleder et al. reported pioneer in vitro and in vivo studies comparing two types of magnetic nanoparticles as potential vascular contrast agents for MRI (Figure 1) [24]. They compared the migration across the capillary wall and the blood half-life of dextran-coated ultra small superparamagnetic iron oxide (USPIO) nanoparticles (2-5 nm diameter) with the nanoparticles AMI-25 [24]. Use of dextran was the key strategy to enhance the uptake and the specificity for macrophages in atherosclerotic plaques in vivo. USPIO nanoparticles had a considerably longer blood half-life with low uptake by the mononuclear phagocytic system (MPS) of the liver and spleen. USPIO nanoparticles showed high permeability through the capillary wall compared to the AMI-25 nanoparticles [24].
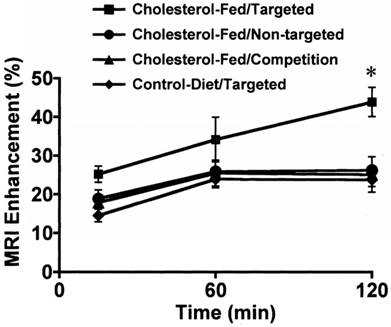
During the 90´s, SPION were coated with different molecules to improve their bioavailability and interaction with specific tissues, for instance, polyclonal human immunoglobulin-labeled iron oxide nanoparticles were evaluated to detect focal inflammation [25]. In the late 90´s, USPIO were used as a contrast agent in magnetic resonance angiography (MRA) due to the T1 shortening effect and their long intravascular half-life, thus improving the visualization of arteries and veins from the thoracic region [42]. At the end of the 90´s and early 00´s, other types of nanoparticles were used for MRI. For instance, a lipid‑encapsulated perfluorocarbon emulsion with gadolinium targeted by fibrin, and a peptidomimetic vitronectin antagonist (αvβ3-integrin targeter) ligand were developed to detect thrombus and the evolution of the vulnerable atherosclerotic plaques (Figure 2) [26-29]. It is worth noting that integrin ανβ3 is only expressed in the angiogenic vasculature, and not in the mature vasculature; hence, it can act as a marker of active angiogenesis associated with plaque progression and with the subsequent myocardium infarction. USPIO nanoparticles were also used as a marker for atherosclerosis‑associated inflammatory changes in the vessel wall before the luminal narrowing [43].
Quantitative analysis of MRI signal enhancement from aorta after treatment with αvβ3-targeted or nontargeted nanoparticles in cholesterol-fed or control diet groups (p˂0.05). Adapted with permission from [28], copyright 2003.
Kooi et al. investigated if USPIO-enhanced MRI could be used for in vivo detection of macrophages in human plaques [44]. To evaluate this, the USPIO were administrated to 11 patients with symptoms of recurrent transient ischemic attacks or small brain infarcts and ultrasound-proven carotid stenosis between 70% and 99% who were scheduled for carotid endarterectomy [44]. The USPIO were detected in macrophages inside the plaques in 10 of the 11 patients and caused a signal decrease on the in vivo MRI images [44]. Using the same strategy, Morishige et al. used 3-T MRI with a macrophage-targeted superparamagnetic nanoparticle preparation (MION-47) in cholesterol-fed New Zealand White rabbits six months after balloon injury [45]. In this study, using in vivo, ex vivo and in situ approaches, the authors demonstrated that the reduction of T2-SI by MION-47 was an indirect measure of the macrophage accumulation in the aorta of hypercholesterolemic rabbits [45].
The evolution of contrast agents for MRI continued with the development of high-density lipoprotein (HDL)-like nanoparticles that exhibit an intrinsic affinity for atherosclerotic plaque macrophages due to the monolayer of apolipoproteins (ApoA‑I or ApoA-II). These HDL-like particles have several advantages, such as: (a) small size (7-12 nm diameter), (b) protein components that are endogenous, biodegradable, and do not trigger immunoreactions, and (c) the particles are not recognized by the mononuclear phagocyte system (MPS) [30]. Furthermore, HDL-like particles are easily reconstituted and can carry a considerable payload of a contrast agent such as phospholipid-based gadolinium (Gd-DTPA-DMPE) as described by Frias at al. [30].
Recent investigations continue to use metal nanoparticles for imaging or targeted treatment of atherosclerosis. Tarin et al. prepared targeted gold-coated iron oxide nanoparticles for CD163 detection in atherosclerosis by MRI [46]. This targeting approach is based on the increased expression of the membrane receptor CD163 in macrophages from intraplaque hemorrhagic sites or asymptomatic plaques. Gold-coated iron oxide nanoparticles conjugated with an anti-CD163 antibody accumulated over time in the atherosclerotic lesions of apoE-deficient mice [46].
Computed tomography
CT is considered the most clinically robust and accurate method for grading coronary artery stenosis and determining plaque calcification. Nonetheless, the specificity and the contrast can be improved using nanoparticles with unique properties. For instance, Hyafil et al. developed iodinated polymer nanoparticles for CT imaging of macrophages in the coronary atherosclerotic plaques [31]. Macrophages play a fundamental role in acute plaque destabilization and thrombus formation by secreting proteases that digest the extracellular matrix and weaken the protective fibrous cap of the atheromatous core. Subsequently, large amounts of tissue factor are released in the atherosclerotic plaques that accelerate thrombus formation and the subsequent plaque rupture [31]. Therefore, it is essential to monitor macrophages as a tool for early detection. Before the injection of the contrast agent, the atherosclerotic plaques could not be differentiated from the surrounding tissues, whereas a strong enhancement was detected in the plaques after the injection of the iodinated nanoparticles, which was not possible with the conventional contrast agents [31]. The same year, Kim et al. prepared a new CT contrast agent based on gold nanoparticles (GNP) coated with polyethylene glycol (PEG) to improve the limitations of contrast agents as an iodine-based compound (renal clearance, renal toxicity, and vascular permeation) [47]. The authors observed that CT images of rats using PEG-coated GNP showed a clear delineation of cardiac ventricles and great vessels [47].
Using high-density lipoprotein that is specific for macrophages (Au-HDL), Cormode et al. detected macrophages in the arteries of atherosclerotic mice and simultaneously imaged the vasculature [4]. The authors showed the capability of the spectral CT system to detect the accumulation of Au-HDL nanoparticles in the aorta using a model for atherosclerosis with apolipoprotein E knockout mice. This result is an indirect evaluation of macrophages in the plaques as it was confirmed by TEM and confocal microscopy that macrophages incorporate the Au-HDL nanoparticles.
Other diagnostic techniques and multimodal imaging
Another imaging technique that benefits from nanoparticles as contrast agents is the photoacoustic imaging, which detects the distribution of optical absorption within the organs. The contrast of a photoacoustic image could be improved using GNP due to the oscillation of free charges on the GNP surface with the excitation wavelength (localized surface plasmon resonance), producing a large optical absorption and an enhanced contrast [48]. For instance, Kim et al. showed the feasibility of gold nanorods (GNR) conjugated to anti-intercellular adhesion molecule-1 (ICAM-1) to detect early inflammation in endothelial cells, which could be related to the evolution of the atherosclerotic plaques [32]. In another example, Wang et al. loaded macrophages with aggregated gold nanoparticles to target atherosclerotic plaques [49]. When plasmon resonance coupling (around 700 nm wavelength) was used, the intravascular photoacoustic technique could detect the presence of aggregated nanoparticles inside the macrophages in the atherosclerotic plaques, providing improved resolution.
Since these diagnostic imaging techniques have limitations, multimodal nanosystems provide an exciting approach to overcome these limitations. The aim is to combine the properties of different nanoparticles by forming hybrid nanosystems with a combination of imaging techniques for improved detection. For instance, PET-CT and PET-MRI are techniques that combine the sensitivity of PET for metabolism imaging and tracking of labeled cells or cell receptors with the outstanding structural and functional characterization of tissues by MRI and the anatomical precision of CT. To meet this challenge, Kelly et al. prepared CVHSPNKKCGGSK(FITC)GK-modified magneto‑fluorescent nanoparticles, which showed high affinity for endothelial cells expressing vascular cell adhesion molecule-1 (VCAM-1) and were detectable by MRI and fluorescence imaging [33]. The VCAM-1 expression is induced early in human atheroma and represents a key element of the inflammation generated during atherosclerosis, contributing to monocyte and lymphocyte recruitment from adventitial vessels and the arterial lumen. The strict temporal and spatial regulation of endothelial adhesion molecules and their critical function in atherosclerosis make them ideal targets for diagnosis [33].
Lipid nanoparticles, such as liposomes and HDL-like nanoparticles, play an important role in imaging and therapy of atherosclerosis. These lipid nanoparticles have been labeled with contrast agents and successfully employed in multimodal molecular imaging. Furthermore, the hydrophobic and hydrophilic core of HDL and liposomes have been loaded with relevant therapeutic agents. Cormode et al. developed multimodality HDL-mimicking nanoparticles by the incorporation of gold, iron oxide, or quantum dot nanocrystals for CT, MRI, and fluorescence imaging, respectively [34]. Gadolinium and rhodamine were also incorporated into nanosystems to get multimodal properties. By using confocal microscopy (CM), transmission electron microscopy (TEM), CT, T1-weighted MRI, T2-weighted MRI, and fluorescence imaging, this study demonstrated effective in vitro uptake of the HDL-like nanoparticles by macrophages. The in vivo applicability of the targeted nanocrystals of HDL for multimodality imaging of atherosclerosis was also demonstrated. Other researchers developed dextran-coated magnetofluorescent iron oxide nanoparticles labeled with the PET tracer 64Cu and a near-infrared fluorochrome to yield a PET-, MRI-, and optically detectable imaging agent [35]. Using these nanoparticles, it was possible to characterize by molecular and cell imaging the evolution of atherosclerotic plaques through analysis of the macrophages involved in inflammation [35].
In recent years, it was shown that inflammatory biomarkers could be used as an effective signal for early diagnosis of atherosclerosis. Pissuwan et al. employed a surface-enhanced Raman scattering (SERS) GNR probe as a tool for the early detection of inflammatory molecules in cells [50]. In this study, the GNR SERS probe detected significant differences in the expression of ICAM-1 in lipopolysaccharide (LPS)-treated macrophages compared to that in untreated macrophages [50]. In contrast, when fluorescent labeling or enzyme-linked immunosorbent assays (ELISA) was used to detect ICAM-1, no significant difference between inflamed and uninflamed macrophages was observed [50].
Nanoparticles for therapy of atherosclerosis
In the nanotechnology field, research on drug delivery systems is a promising area. Using nanotechnology strategies, it is possible to combine sustained release of the drug with targeting systems for delivery in specific tissues or targets.
Nanoparticles as vehicles for drug delivery
In addition to systemic administration, current materials or devices can also be improved with drug delivery nanosystems. For instance, polymer nanoparticles have been used for drug delivery to treat restenosis after the percutaneous coronary intervention. In this study, polymer nanosystems composed of core-shell nanoparticles of polyethyleneglycol-based block copolymers encapsulating doxorubicin as an antiproliferative drug were selectively released to the balloon-injured artery [36]. Polymer nanoparticles were also used as drug delivery systems from the surface of the stents by Nakano et al., who developed bio-absorbable polymeric nanoparticles of poly(lactic-co-glycolic acid) (PLGA) encapsulating a fluorescent marker, fluorescein isothiocyanate (FITC) [37]. This drug delivery nanosystem was used to coat the surface of the stainless-steel balloon-expandable stents. Compared with the dip-coated polymer-eluting stent, this nanoparticle-eluting stent system showed unique aspects of novel electrodeposition coating technology, vascular compatibility, and a better and more prolonged delivery of the FITC marker into the stented porcine coronary artery [37].
Leuschner et al. developed liposomes encapsulated with a small interfering ribonucleic acid (siRNA) to prevent the accumulation of monocytes in atherosclerotic plaques [38]. Since inflammatory monocytes depend on the chemokine receptor CCR2 for localization to injured tissues, siRNA released from the liposomes silenced the CCR2 receptor, reducing the number of monocytes/macrophages accumulating in inflammatory atherosclerosis by 82% [38]. In another instance, Duivenvoorden et al. developed reconstituted high-density lipoprotein nanoparticles (rHDL) loaded with a statin to inhibit inflammation in atherosclerotic plaques [39]. This study showed the potent anti-inflammatory effect of the statin-rHDL treatment to inhibit the plaque inflammation progression when administered using a three-month low‑dose regimen, while a one-week high-dose regimen markedly decreased inflammation in advanced atherosclerotic plaques [39].
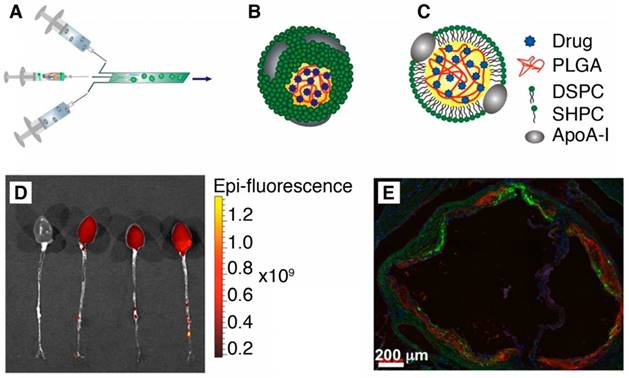
Recently, the second generation of HDL nanoparticles was developed by incorporating PLGA polymer inside its hydrophobic core to allow the controlled release of drugs [40]. These hybrid PLGA/HDL biomimetic nanoparticles produced by microfluidics technology showed preferential uptake by macrophages due to the intrinsic characteristic of the HDL combined with a slow release profile of a hydrophobic dye from PLGA (Figure 3). Also, in vivo studies using an ApoE knockout mouse model of atherosclerosis showed clear accumulation of these PLGA-HDL nanoparticles in atherosclerotic plaques [40].
(A) Scheme of the synthesis of PLGA-HDL by microfluidic technology. (B-C) 3D and 2D schematics of the nanoparticle structure. (D) Targeting to atherosclerotic plaques. Fluorescence imaging of excised aortas of ApoE-KO mice injected with placebo or PLGA-HDL nanoparticles. Uptake can be seen throughout the thoracic and abdominal aorta as well as in the aortic root of the PLGA‑HDL injected mouse. (E) A stitched 20x fluorescence microscopy image is shown of the aortic root (scale bar, 200 µm); red signifies a stain for CD68, green PLGA-HDL and blue cell nuclei (DAPI). Adapted with permission from [40], copyright 2015.
Nanoparticles with active targeting
Innovation in nanoparticles to treat atherosclerosis includes improving the blood half-life, vascular margination, and active targeting. Platelets can marginate to the vascular wall and specifically interact with vascular injury sites favored by their shape, flexibility, and complex surface interaction. Inspired by this, Anselmo et al. reported nanoparticles that exhibited platelet-like characteristics including injury-specific margination and adhesion and amplification of injury-specific aggregation [51]. These platelet-like nanoparticles (PLN) with discoidal morphology and mechanical flexibility, exhibited enhanced surface-binding compared to those with spherical and rigid discoidal morphology [51]. Moreover, site-selective adhesive and platelet-aggregatory properties under physiological flow conditions in vitro were obtained. In vivo studies showed that PLN accumulated at the wound site and induced around 65% reduction in bleeding time, effectively mimicking and improving the hemostatic functions of natural platelets [51].
A study conducted by Kheirolomoom et al. developed another interesting approach of active targeting to atherosclerotic plaques based on cationic lipoparticles (CCL) containing anti-miR-712 within the core and with a neutral coating decorated with a peptide (VHPKQHRGGSKGC) to target VCAM-1 [52]. Optical imaging validated disease-specific accumulation, as anti-miR-712 was efficiently delivered to inflamed mouse aortic endothelial cells in vitro and in vivo. Furthermore, an 80% lower dose of VHPK-CCL-anti-miR-712 as compared with naked anti-miR-712 prevented atheroma formation in a mouse model of atherosclerosis [52]. Recently, gold nanospheres (GNS) with VCAM-1-binding peptide (VHSPNKKGGSKGC) targeted to inflamed endothelium were also used as a vehicle for anti-miR-712 to inhibit the formation of atherosclerotic plaques [53].
Historical review of nanoparticles used in diagnosis and therapy of myocardial infarction
The evolution of the use of nanotechnology in diagnosis and therapy of MI is shown in the timeline scheme in Figure 4. The application of nanoparticles for MI began around the 90's with the use of SPION for MRI [54]. Targeted magneto-fluorescent nanoparticles and other hybrid nanoparticles based on iron oxide and gold have been used to diagnose, by multimodal imaging techniques, different processes involved in the MI such as cardiomyocyte apoptosis and phagocyte recruitment [55,56]. From early 00's to date, polymer nanosystems, micelles, liposomes, extracellular vesicles, and lipid nanoparticles have been used for developing drug delivery strategies. The aim was to counteract a variety of pathological processes related to ischemia including the oxidative stress, inflammation, cardiomyocyte apoptosis, and ischemia/reperfusion injury, thus attenuating the post-MI pathological remodeling and avoiding heart failure [57-61].
Timeline of nanotechnology for diagnosis and therapy of myocardial infarction.
Early detection of cardiac biomarkers, such as the cardiac troponin I (cTnI) released into the bloodstream during the myocardial damage process, has become important to decrease the risk of death. Around 2009, a study reported the use of highly sensitive biosensors using GNRnan [62]. Other conductive nanoparticles such as graphene and carbon nanotubes have also been used. Biosensors with increased sensitivity using nanoparticles are increasingly being used for the detection of relevant cardiac biomarkers such as cTn1, cTnT, myoglobin, and glutathione [63-65].
Following cardiac ischemia, regeneration strategies based on nanotechnology have been used in the last two decades. For instance, nanoparticles releasing growth factors or miRNA were employed to promote angiogenesis [66]. Also, scaffolds with conductive nanoparticles or nanostructured patches to support and stimulate the growth of stem cells and subsequent formation of new tissue are examples of other approaches explored for cardiac regeneration after MI [67-72].
During the last several years, SPION continued to play a significant role in cardiac diagnosis by MRI. New functionalities and targeting molecules have been explored to improve their selectivity and sensitivity in the detection of biochemical and cellular processes during MI [73].
A more detailed description of the studies that have developed nanosystems for the diagnosis and treatment of MI is given below.
Nanoparticles for diagnosis of myocardial infarction
For cardiac imaging, contrast agents are necessary to differentiate the myocardium tissue from the heart lumen [74]. A chronological description of studies on nanoparticles used in MRI, CT, and multimodal imaging as well as in the biomarkers detection applied to MI is presented below.
Magnetic resonance imaging
The advantages of cardiac MRI include its tomographic nature, high spatial and temporal resolution, and excellent soft tissue contrast. The first nanoparticles to be used for MRI of the heart were the superparamagnetic ferrite nanoparticles AMI‑25 employed in studies of myocardial reperfusion reported in 1989 [54]. Signal intensity remained constant after the injection of AMI-25 in the presence of the occlusion and decreased significantly with reperfusion. The signal intensities were similar in different myocardial regions at all stages of occlusion and reperfusion [54]. During the 90´s and early 00´s, SPION was frequently utilized for imaging of the myocardium by MRI. For instance, Chapon et al. used SPION to discriminate between infarcted and normal tissues in rats by high-field MRI (T2-weighted images) [75]. The same year, Kraitchman et al. obtained poly-L-lysine-SPION (Feridex)-mesenchymal stem cells (MSC) to track the MSC magnetically in a swine MI model. MRI tracking of MSC was feasible and represented a preferred method for studying the engraftment of MSC in MI, allowing the evaluation of the enormous therapeutic potential of MSC for limiting infarct size and restoring cardiac function after MI [76]. Amsalem et al. also labeled MSC with SPION to track them in a rat model of MI. At four weeks after the transplantation of SPION-labeled MSC, the authors observed the MRI signal sources from cardiac macrophages that engulfed the SPION nanoparticles [77].
Inflammation following acute MI has detrimental effects on reperfusion, myocardial remodeling, and ventricular function. Alam et al. were pioneers in the use of USPIO nanoparticles to detect the cellular inflammation in human cardiac tissues by MRI. Sixteen patients with acute ST-segment elevation MI were evaluated, acquiring T2-weighted multigradient-echo sequences and R2* values for specific regions of interest. Uptake of USPIO nanoparticles occurred in the infarcted and remote myocardium [78]. Yilmaz et al. showed in humans that a commercial USPIO-based contrast agent (ferumoxytol, FerahemeTM) enabled a more detailed characterization of MI using T2-weighted MRI compared to gadolinium-based compounds, mainly by detecting infiltrating macrophages [79]. Considering the multi-functionality of USPIO-based nanoparticles and their superior safety profile, these observations open up new vistas for the clinical application of USPIO.
Recently, new functionalities of SPION conjugated with recombinant 70 kDa heat shock protein (Hsp70) have been explored [73]. An increased CD40-mediated cellular uptake of Hsp70-SPION nanoparticles was observed compared to non-conjugated SPION. Following the induction of acute infarction in rats, SPION and Hsp70-SPION were injected intravenously, and subsequent MRI and biodistribution analyses indicated the preferential accumulation of the Hsp70-SPION in the cardiac tissue [73].
Computed tomography
Computed tomography is a powerful imaging technique that has several strengths, such as high spatial and temporal resolution, and excellent quantitative capabilities. CT using nanoparticles as contrast agents has been mainly used to evaluate the coronary artery in atherosclerosis. Some studies have evaluated the feasibility of using nanoparticles for CT to evaluate the MI processes in small animals. For instance, Danila et al. used gold nanoparticles functionalized with a peptide that recognized collagen (collagen adhesion, CNA35) to target the myocardial scar [80]. The purpose of this was to provide adequate contrast for CT imaging. Although the small size of the experimental animals (mouse) and the even smaller size of the myocardial scar were a challenge for this method, this study was the first to utilize AuNPs for myocardial scar detection with CT imaging and must be further developed in the future. Nevertheless, the results of this investigation indicated that in the mice with larger scar burden, the use of AuNPs allowed detection of the focal contrast enhancement in the myocardium that was not observed in the control animals [80].
Recently, Sawall et al. developed a pre-clinical micro-CT method using a contrast agent based on nanocapsules with high iodine concentration (ExiTronTM MyoC 8000) to longitudinally monitor cardiac processes in vivo in healthy and MI-inflicted mice [81]. The study also evaluated nanoparticles of high alkaline-earth metal content (ExiTronTM nano) [81]. Due to its ability to be taken up by infarct myocardium but not by healthy tissue, ExiTron MyoC 8000 enabled detection of MI even at a very low dose. The signal enhancement after contrast agent injection was exploited for quantification of infarct size. Thus, the developed micro-CT method allowed monitoring of a variety of processes such as cardiac remodeling in longitudinal studies [81]. In another study, Cormode et al. used AuNPs with an iodinated contrast agent in a new prototype spectral photon-counting computed tomography (SPCCT) system [82]. They found that the contrast material maps clearly differentiated the distributions of gold and iodine in the tissues, allowing quantification of the contrast agents' concentrations, which matched their expected pharmacokinetics [82]. Furthermore, rapid, repetitive scanning was done, which allowed measurement of contrast agent kinetics with high temporal resolution. In conclusion, a clinical scale, high count rate SPCCT system was able to discriminate between gold and iodine contrast media in different organs in vivo, including the left ventricle [82]. Such rapidity of scanning has not been demonstrated to be able to assess arterial input function for potential quantification of abnormal tissue perfusion, as in the case of MI [82].
Other diagnostic techniques and multimodal imaging
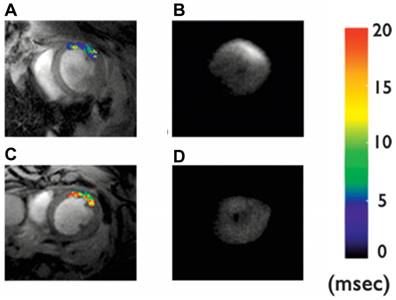
Cardiomyocyte apoptosis has been implicated in numerous cardiac diseases such as ischemia, reperfusion injury, and heart failure. However, the absence of a reliable biomarker to detect and characterize cardiomyocyte apoptosis in vivo has hindered the translation of this basic molecular understanding into clinical practice. A highly suitable ligand for targeted apoptosis imaging is Annexin V, which binds selectively and with a high affinity to the phosphatidylserine expressed on the membrane of apoptotic cells. Sosnovik et al. showed the feasibility of imaging cardiomyocyte apoptosis in vivo by high-resolution MRI and ex vivo by fluorescence imaging using annexin V-labeled magnetic and fluorescent nanoparticles (Annexin V-cross-linked iron oxide-Cy5.5 nanoparticles, AnxCLIO-Cy5.5) [55] (Figure 5).
In vivo T2* maps and corresponding ex vivo near-infrared fluorescence images of mice given Anx-CLIO-Cy5.5 (A-B) and CLIO-Cy5.5 (C-D). T2* maps were created in a region-of-interest (ROI) in the myocardium, defined by the zone of hypokinesis. A significant reduction in myocardial T2* values was seen in the ROI of mice given the Anx-CLIO-Cy5.5 probe (A) vs. those given the CLIO-Cy5.5 probe (C). NIRF images of the excised hearts revealed a corresponding increase in fluorescence intensity in the mice given Anx-CLIO-Cy5.5 (B), but not in those given CLIO-Cy5.5 (D). Adapted with permission from [55], copyright 2005.
Another magneto-fluorescent nanoparticle CLIO-VT750 was employed by Nahrendorf et al. for imaging of phagocyte recruitment as a multimodal tool to serially and noninvasively assess cellular and molecular functions in post-infarct inflammation [56]. They used multichannel fluorescence molecular tomography (FMT)-CT to detect impaired recruitment of phagocytes and protease activity mediated by macrophages and neutrophils in murine infarcts [56]. FMT-CT with CLIO-VT750 nanoparticles was shown to be a promising noninvasive molecular imaging approach to characterize infarct healing [56].
During the myocardial damage process, the cardiac troponin I (cTnI) is released into the bloodstream. Early detection of cTnI in the serum of patients with higher risk of MI can decrease the risk of death. For instance, Guo et al. developed a facile and rapid solution-phase method to detect human cTnI using anti-human cTnI-labeled GNR-based biosensors [62]. Sensing with a detection limit of 10 ng/mL was shown by the change in the plasmon resonance wavelength of the GNR with specific antibody‑antigen binding events [62].
In the last few years, nanoparticles such as GNP, carbon dots, and graphene have continued to be key protagonists of different types of sensors (e.g., electrogenerated chemiluminescence sensor) to enable multiple and ultrasensitive detection of relevant cardiac biomarkers as cTn1, cTnT, myoglobin, and glutathione [63-65].
Nanoparticles for therapy of myocardial infarction
Nanoparticles for therapy of MI have been used as vehicles to deliver therapeutic agents to mitigate inflammation, oxidative stress, and necrosis, among other processes. Furthermore, some nanocomposites and templates (e.g., scaffolds or patches) have been developed to support cell growth and promote cardiac regeneration.
Nanoparticles as vehicles for drug delivery
Lukyanov et al. prepared micelles of polyethyleneglycol/phosphatidyl-ethanolamine conjugates (PEG-PE) with a size of 7-20 nm, which were administered into rabbits with MI [57]. These micelles showed a prolonged circulation time in blood (half-life of 2 h) and accumulated in the infarction zone with 8-fold higher efficiency compared to a non-damaged part of the heart muscle [57]. These results suggested that the PEG-PE micelles can be used for the delivery of drugs due to their accumulation in the infarct areas mediated by the enhanced permeability and retention (EPR) effect due to the inflammatory process.
Inflammation and oxidative stress have been key players in ischemic heart disease. Oxidative stress due to excessive production of reactive oxygen species (ROS) plays a key role in the pathogenesis of cardiac remodeling leading to heart failure. Cerium oxide (CeO2) nanoparticles have shown protection of cells in culture from oxidative stress. For instance, Niu et al. assessed the effects of CeO2 nanoparticles on cardiac function and remodeling as well as the endoplasmatic reticulum (ER) response in a murine model of cardiomyopathy. CeO2 nanoparticles protected against the progression of cardiac dysfunction and dilatation by attenuating the myocardial oxidative stress, ER stress, and inflammatory processes [83].
Nanoparticles with RNA have also been used to counteract the oxidative stress produced after MI. For instance, the CCR2 receptor, which governs inflammatory Ly-6C high monocyte subset traffic, was silenced by RNA-loaded lipid nanoparticles attenuating the infarct inflammation and the post-MI left ventricular remodeling [58]. Another important gene silenced by a complex of siRNA and low molecular weight polyethyleneimine modified with deoxycholic acid (PEI-DA) was the Src homology region 2 domain-containing tyrosine phosphatase-1 (SHP-1). In this instance, the PEI‑DA/siRNA complexes silenced the SHP-1 gene expression in cardiomyocytes, which led to a significant inhibition of cardiomyocyte apoptosis under hypoxia [59].
Currently, the use of engineered extracellular vesicles (EV) for the treatment of cardiac diseases is being investigated [84-87]. EV, especially their exosome fraction, are a key component of paracrine secretion in many cell types. Exosomes are 40-150 nm-sized particles stored intracellularly in endosomal compartments, which are secreted for cell communication. Barile et al. isolated exosomes from cardiac progenitor cells (CPC) and injected them in infarcted heart, thus leading to decreased cardiomyocyte apoptosis, enhanced angiogenesis, and improved ejection fraction compared with hearts injected with control medium [60].
Recently, ischemia-reperfusion (IR) injury was treated in a preclinical porcine model with PLGA nanoparticles loaded with pitavastatin (3-hydroxy-3-methylglutaryl coenzyme-A reductase inhibitor), which showed a cardioprotective effect associated with activation of the PI3K-Akt pathway and reduced inflammation [61]. Transplantation of gene‑transfected bone marrow-derived mesenchymal stem cells (BMMSC) is also a promising strategy for ischemic myocardium repair. However, the current therapeutic strategy suffers from high toxicity and inefficient gene transfection. To overcome this challenge, Zhu et al. synthesized organic-inorganic hybrid hollow mesoporous organosilica nanoparticles (HMONs) loaded with the hepatocyte growth factor (HGF) gene to transfect BMMSC. HGF gene-transfected BMMSC decreased apoptotic cardiomyocytes, reduced infarct scar size, relieved interstitial fibrosis, and increased angiogenesis in the myocardium, thus improving myocardial function [88].
Regenerative therapy and scaffolds
The promotion of angiogenesis is essential for the regeneration of the cardiac tissue after ischemia. For instance, Sang Oh et al. [67] prepared vascular endothelial growth factor (VEGF)-loaded core/shell nanoparticles for the regeneration of ischemic heart. The core of these nanoparticles was composed of lecithin-containing VEGF and the shell of the Pluronic® F-127 triblock copolymer. The results showed that these VEGF-loaded nanoparticles improved heart functions, in particular, the ejection fraction and cardiac output [66].
Other researchers designed nanostructures to mimic the strong angiogenic signal of the VEGF protein [89]. These VEGF-mimicking nanostructures were obtained by self-assembly of peptide amphiphiles forming nanoscale filaments that displayed a VEGF-mimetic peptide on their surface. These VEGF-mimetic filaments induced phosphorylation of VEGF receptors and promoted proangiogenic behavior in endothelial cells. In vivo studies elicited an angiogenic response in the host vasculature. When the nanofibers were evaluated in a mouse hind-limb ischemia model, increased tissue perfusion and functional recovery were observed, illustrating a promising synthetic therapeutic strategy to regenerate the microcirculation and restore perfusion to ischemic tissue [89]. Another strategy to foster cardiac regeneration after ischemia or heart attack is the use of porous matrices called scaffolds, which provide mechanical support for the cellular and vascular growth. In the last decade, several approaches based on scaffolds have been developed. For instance, Dvir et al. showed that incorporating gold nanowires within alginate scaffolds could bridge the alginate pore walls and thus improve the electrical communication between adjacent cardiac cells [67]. Cardiac tissue grown on these composite scaffolds was thicker and better aligned than that grown on the alginate matrix without gold, and when the composite scaffold was electrically stimulated, the cells in these tissues contracted synchronously [67]. A similar study prepared a conductive scaffold with Young's moduli similar to the myocardium based on a porous matrix of hydroxyethyl methacrylate with GNP [68]. The study showed that the connexin 43 protein was upregulated in cardiomyocytes seeded into the conductive scaffolds, stimulated electrical signaling between cells, and improved the cardiomyocyte function [68]. Carbon nanotubes (CNT) were also used to give conductive cues to polymer scaffolds used in cardiac regeneration. Mooney et al. prepared electrically stimulated MSC exposed to medium containing CNT and seeded on CNT/polylactic acid scaffolds [69]. After electrical stimulation, the cells reoriented perpendicular to the direction of the electrical current and adopted an elongated morphology. Moreover, an upregulation of the cardiac markers such as myosin heavy chain, cardiac homeobox protein (Nkx2.5), transcription factor GATA-4, cardiac troponin T (cTnT), and connexin43 was detected [69].
Another way to foster the formation of a stem cell niche and stimulate myocardial regeneration using nanotechnology is through the design of a nanopattern on artificial matrices used as cardiac patches. Kim et al. used autologous cardiosphere-derived cells (CDC) with nanotopographically defined hydrogels, mimicking the native myocardial matrix to form cardiac stem cell niches and control the cell function and differentiation [70]. Highly anisotropic and controlled augmentation of cell adhesion, migration, and proliferation was obtained in cell cultures. Using a rat infarction model, engraftment of nanofabricated patches with CDC enhanced retention and growth of the transplanted cells, and their integration with the host tissue was observed [70].
Injectable GG' hydrogel for MI therapy. (A) Schematic of the stepwise formulation process of nanobioactive hydrogel and subsequent injection to treat damaged heart with acute myocardial infarction. (B) Scar area determination by morphometric analysis of the left ventricle in different groups. (C) Echocardiographic assessment of cardiac function. For this, ejection fraction (EF%) was monitored at day 2 and 14 post treatment. GG' showed significantly better EF% than other groups. Data are expressed as mean value ± SD. ANOVA statistical analysis with Bonferroni post-hoc test was performed. ****P < 0.0001; ***P < 0.001, **P < 0.01, *P < 0.05 vs. time-matched control (n = 7). P values comparing time-matched GG' and GG are indicated by ψ. Adapted with permission from [72], copyright 2014.
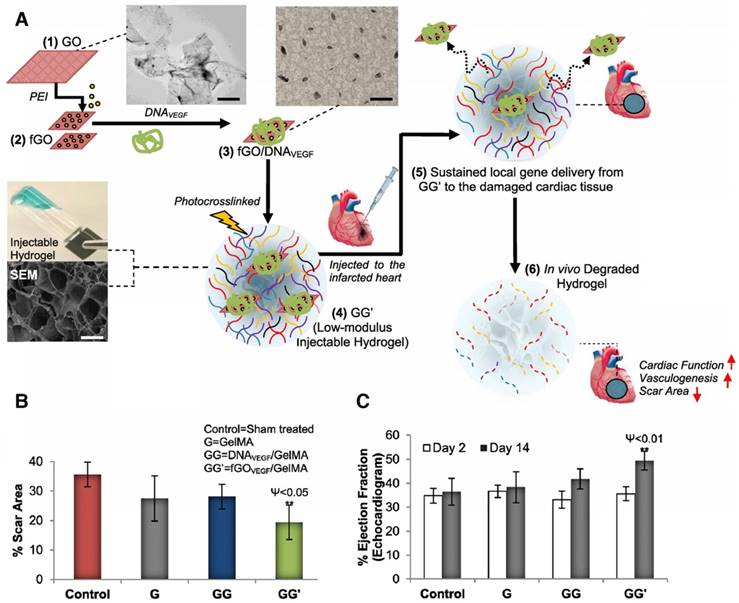
Another GNP/scaffold with improved conductivity was developed by Shevach et al. by depositing GNP on fibrous decellularized matrices [71]. The gold-hybrid scaffolds exhibited a superior function than pristine patches as cardiac cells had elongated and aligned morphology and organized electrical coupling proteins (connexin 43) [71]. Strategies with injectable hydrogels for myocardial therapy have also been explored using low-modulus methacrylated gelatin (GelMA), which could efficiently deliver a nanocomplex of polyethyleneimine (PEI) functionalized with graphene oxide (fGO) and a VEGF pro-angiogenic gene (Figure 6A) [72]. In vitro transfected cardiomyocytes secreted VEGF and exhibited profound mitotic activities on endothelial cells [72]. fGOVEGF/GelMA hydrogels, which were injected in the peri-infarct region, reduced the scar area in the infarcted heart compared to those of the untreated sham, GelMA, or DNAVEGF/GelMA groups (Figure 6B). Furthermore, the fGOVEGF/GelMA group showed significantly higher cardiac performance by echocardiography (Figure 6C) [72]. Other types of nanoparticles have been incorporated into scaffolds in combination with other therapeutic strategies such as stem cells and therapeutic molecules for cardiac regeneration. Another strategy based on nanoparticles and polymer scaffolds was evaluated recently to stimulate angiogenesis and differentiation of stem cells into cardiomyocyte lineage by incorporating bioactive glass nanoparticles (nBG) into gelatin-collagen hydrogels [90]. The findings suggested that nBG inclusion in a hydrogel scaffold promoted angiogenesis by increasing VEGF secretion level, and induced differentiation in human endometrial stem cells (EnSC) toward endothelial lineage [90]. In another study, nanoclays were incorporated into GelMA hydrogels for the controlled release of growth factor-rich conditioned media (secretome) [91]. This nanocomposite scaffold provided a dual action therapeutic system through its pro-angiogenic and cardioprotective capacity providing an alternative to stem cell therapy for the cardiac regeneration after MI [91].
The findings described above show the evolution of nanoparticles since the end of the 80´s, revolutionizing the possibilities of diagnostic and treatment strategies for atherosclerosis and MI. The last years have witnessed the evolution of multifunctional nanoparticles consisting of versatile materials conjugated with organic biomolecules for more specific, precise, and safe diagnosis and treatment.
From diagnosis and therapy to theranostic approaches
The development of multifunctional nanoparticles and new strategies based on nanotechnology have generated new clinical challenges. A significant challenge is the need to assess the long-term effect of the treatment following changes in cellular processes and physico-chemical characteristics of the tissues. Based on their intrinsic properties, nanoparticles (nanocomposites, nanoconjugates, and nanocomplexes, among others) with optical and magnetic properties are used as contrast agents, vehicles for the delivery of therapeutic molecules, or directly in the treatment. The combination of optical and plasmonic properties of GNPs, along with tailored interactions between drugs and polymers, could be useful for combining diagnosis and treatment. These new approaches gave rise to the term “theranostics”, which is described by the National Institutes of Health (NIH) as the application of nanobiomaterials and devices at the molecular level for personalized diagnosis, imaging, and therapy [92]. The term “theranostics” was employed for the first time in the early 00´s [93] and in recent years has become a field with rapid growth and great future. In the cardiovascular field, theranostic strategies based on nanotechnology started to be used in 2006. The timeline shown in Figure 7 depicts some milestones in the use of nanoparticles for atherosclerosis and MI.
Timeline of nanotechnology for theranostic strategies of atherosclerosis and myocardial infarction.
There is a need to diagnose and treat vulnerable plaques in atherosclerosis before clinical manifestations. In particular, the macrophages have emerged as a key biological, imaging, and therapeutic target for atherosclerosis. For instance, Weissleder et al. prepared theranostic nanoparticles based on dextran-coated SPION conjugated with Alexa Fluor 750 [94]. A potent photosensitizer, 5-(4-carboxyphenyl)-10,15,20-triphenyl-2,3-dihydroxychlorine (TPC) was covalently conjugated to the fluorescent magnetic nanoparticles for a phototherapy procedure of the plaque by cytotoxic singlet oxygen. This nanosystem allowed the highly efficient killing of murine and human macrophages in vitro, which was detectable by MRI and near-infrared fluorescence imaging [94]. Another pioneering study of a theranostic strategy was described by Winter et al., who prepared αvβ3 integrin-targeted paramagnetic nanoparticles with the peptidomimetic vitronectin antagonist conjugated to PEG2000 phosphatidylethanolamine [95]. This nanosystem was used for the selective delivery of fumagillin (mycotoxin produced by Aspergillus fumigatus, which suppresses angiogenesis by inhibition of methionine aminopeptidase 2 (Met AP2)) and inhibited angiogenesis in the plaques. Polyak et al. used polymer-coated SPION for magnetic targeting of endothelial cells to the surface of steel stents as a cell therapy strategy for injured arteries [96]. In vivo SPION-loaded bovine aortic endothelial cells transduced with adenoviruses expressing luciferase (Luc) were targeted to the stent under the presence of a uniform magnetic field and showed high Luc expression by in vivo optical imaging [96]. Another theranostic strategy based on magnetic nanoparticles was used by Ma et. al. [97], which consisted of nanoclusters composed of closely spaced gold-coated SPION. These nanoclusters were coated with dextran, allowing high uptake by macrophages. A strong NIR contrast in dark field and hyperspectral microscopy was obtained both in cell cultures and in an in vivo rabbit model of atherosclerosis [97]. In 2010, McCarthy et al. prepared dextran-coated SPION modified with near-infrared fluorophores and light-activated therapeutic moieties to stabilize the atherosclerotic plaques [98]. After intravenous administration, the nanoagent was localized within the macrophage-rich atherosclerotic lesions by fluorescence microscopy. Irradiation of the atheroma led to the phototoxic activation of the therapeutic component and eradication of inflammatory macrophages [98].
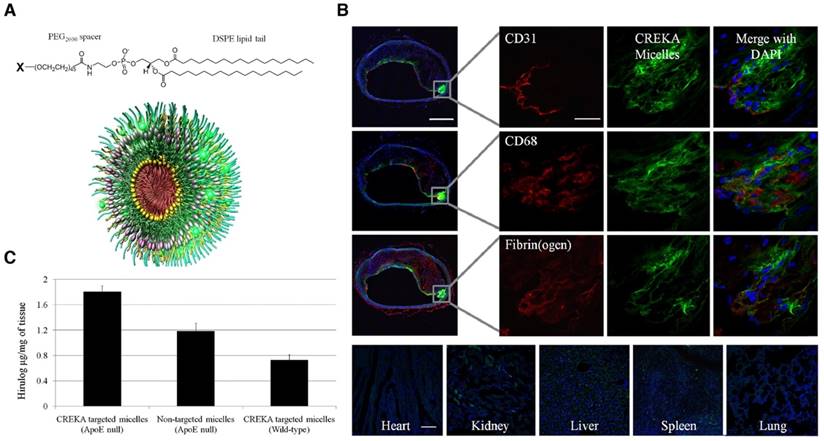
Besides magnetic nanoparticles, micelles have been prepared for theranostics of the atherosclerotic plaques. Peters et al. obtained multifunctional micelles composed of a 1,2-distearoyl-sn-glycero-3-phosphoethano-lamine (DSPE) tail, a PEG2000 spacer, and a variable head group based on the carboxyfluorescein (FAM)- cysteine-arginine-glutamic acid-lysine-alanine (CREKA) peptide, and an infrared fluorophore, or the hirulog peptide (anticoagulant) (Figure 8A) [99]. These CREKA-targeted micelles showed fluorescence and antithrombin activity in the atherosclerotic plaques of ApoE-null mice (Figure 8B-C). As a targeting strategy, the authors also demonstrated the affinity of the CREKA pentapeptide to fibrin-fibronectin complexes in clotted plasma, the residing place of the plaques [99]. The same research group also evaluated in multifunctional nanosystems another relevant 9-amino acid cyclic peptide, LyP-1 with binding ability to atheroma-associated macrophages in plaques [100,101].
Other nanoparticles used for photothermal ablation of macrophages in atherosclerotic plaques were single wall carbon nanotubes (SWNT) functionalized with Cy5.5 fluorescent dye (Cy5.5-SWNT) [102]. Cy5.5-SWNT showed higher signal intensity in ligated carotids compared with sham by near-infrared fluorescence imaging. Light (808 nm) exposure of carotids with SWNT showed heat-induced apoptosis in macrophages, which was absent in control groups without SWNT or light exposure [102].
The pathogenesis of ischemia/reperfusion (I/R) injury is characterized by the generation of a high level of hydrogen peroxide (H2O2). For instance, Lee et al. prepared polyoxalate copolymer nanoparticles (HPOX) as a novel diagnostic and therapeutic strategy for I/R injury [103]. These nanoparticles had the ability to react with H2O2 to perform peroxalate chemiluminescence reaction in the presence of fluorescent compounds. Furthermore, 4-amino-1,8-napthalimide (4-AN) was incorporated into the nanoparticles as a model drug. HPOX nanoparticles generated a robust image of H2O2 in the hind limb IR injury and effectively released the anti-apoptotic drug after I/R injury, exhibiting their effectiveness as an imaging agent and drug delivery system [103]. Another type of nanoparticle with the ability to react with reactive oxygen species (ROS) in macrophages was a chlorin e6 (Ce6)-hyaluronic acid (HA) conjugate (Ce6-HA), which became highly fluorescent and phototoxic when reacting with ROS, especially peroxynitrite. Upon illumination, Ce6-HA nanoparticles showed significant potential for selective NIR fluorescence imaging and highly phototoxic effect on macrophages [104].
Multifunctional micelles. (A) micelles were composed of individual monomers made up of a DSPE tail, a PEG2000 spacer, and a polar head group (X) of CREKA, FAM-CREKA, FAM, N-acetylcysteine, Cy7, or hirulog drug. (B) Localization of CREKA micelles in atherosclerotic plaques. Serial cross-sections were stained with antibodies against CD31 (endothelial cells), CD68 (macrophages and other lymphocytes), and fibrin (ogen). Micelles were bound to the entire plaque surface with no apparent binding to the healthy region of the vessel. CREKA-targeted micelles also penetrated under the endothelial layer (CD31) where there was high inflammation (CD68) and the plaque was prone to rupture. Clotted plasma proteins were seen throughout the plaque and its surface (fibrin(ogen) staining). Fluorescence was not evident in the heart or lung, and slightly in the kidney, spleen, and liver. (C) Targeting of hirulog to atherosclerotic plaques. Significantly higher levels of antithrombin activity were observed in the aortic tree of ApoE-null mice after injection of CREKA-targeted hirulog micelles than that of nontargeted micelles. Adapted with permission from [99], copyright 2009.
Cheng et al. prepared imageable SPION conjugated to two different types of antibodies: one was to facilitate in vivo binding of the particles to the injured myocardium and the other was for the subsequent capture and localization of endogenous or exogenous therapeutic cells. MRI, fluorescence microscopy, and the echocardiography showed that these bifunctional nanoparticles improved the localization of exogenous therapeutic cells in myocardial infarcted rat, and helped to preserve the structure and cardiac function post-infarction. Application of an external magnetic field to attract the superparamagnetic nanoparticles allowed localization of the nanoparticles and improved the cardiac function [105].
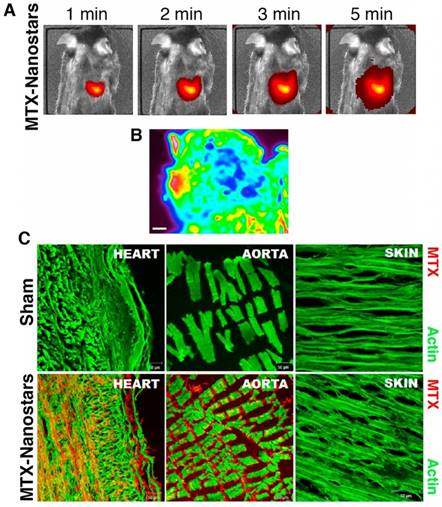
Hybrid lipid-latex (LiLa) nanoparticles with lipids bearing phagocytic signals or “eat me” signals were prepared by Bagalkot et al. as an effective strategy to improve the targeting and uptake of nanoparticles by macrophages [106]. These LiLa nanoparticles were self-assembled with anti-inflammatory drugs, gadolinium, and fluorescein. In a mouse model of atherosclerosis, LiLa nanoparticles targeted macrophages, allowing noninvasive imaging of atherosclerotic plaques by MRI as well as treatment [106]. GNP have also been developed as a theranostic strategy for atherosclerotic plaques. In recent years, GNR and gold-silica nanoparticles were used for photothermal ablation and optical imaging of macrophages, showing high cell-killing efficacy after NIR irradiation [107,108]. Additionally, Simian virus 40 (SV40)-based nanoparticles with near-infrared quantum dots, a targeting element (peptides for VCAM-1, macrophages, and fibrin), and a drug component (anticoagulant drug Hirulog) were prepared for targeted imaging and therapy of atherosclerosis [109]. These SV40-based nanoparticles were a suitable theranostic platform for in vivo optical imaging, molecular targeting, and drug delivery in atherosclerosis [109]. In another theranostic example, gold nanostars conjugated to the drug mitoxantrone (MTX) were used for SERS of cancer cells, and for delivery of the antitumoral drug, which had shown a nonspecific anti-inflammatory effect for patients with CVD [110]. After intravenous administration, gold nanostars massively accumulated in the heart of healthy mice (Figure 9A) due to the presence of a highly organized vascular structure in the heart favoring the accumulation of drugs. Absorbance FTIR maps also showed the high intensity of MTX-nanostars in the heart tissue (Figure 9B). The multiple vertices of the nanostars could enhance the MTX signal in the infrared range, thus providing the location and density of MTX-nanostars in cardiac tissue. Consequently, the experimental setting could reduce laboratory operations and maintenance costs. This method proved to be effective in detecting drug accumulation, which is relevant for controlling spatial and temporal drug release. Confocal microscopy images and immunolocalization staining of the heart and aorta tissues confirmed that the MTX-nanostars could cross the endothelial membrane (Figure 9C) [110]. These results confirmed great potential of this theranostic strategy for patients with CVD.
In vivo images of MTX-gold nanostars in a mouse model. (A) In vivo live images of mice (n=6 animals) 1 min to 5 min after IV injection of MTX-gold nanostars. (B) Absorbance FTIR maps from the heart tissue. Red color represents the area with nanoparticles, green and blue light indicate background (scale bar, 200 µm). (C) Confocal microscopy images of heart and aorta tissue immunostaining from sham (no treatment) and MTX-gold nanostar-treated groups, showing the biodistribution and accumulation of the nanosystems in the heart and aorta endothelium. Red color represents the region with MTX-gold nanostars and green color shows actin staining. Adapted with permission from [110], copyright 2016.
Transplantation of autologous mitochondria to the myocardial ischemic zone is expected to augment or replace the function of the damaged mitochondria and allow for enhanced post-ischemic functional recovery and preservation of cell viability. Cowan et al. utilized SPION and 18F-rhodamine to deliver and visualize (PET, CT and MRI) exogenous mitochondria in the injured heart. The nanoparticle-based delivery of autologous mitochondria through the coronary vasculature significantly decreased infarct size and enhanced post-ischemic myocardial function [111].
Cardiac patches have also evolved for theranostic approaches. For instance, Feiner et al. developed hybrid cardiac patches with multifunctional electronics for online monitoring and regulation of tissue function. These engineered cardiac patches integrated cardiac cells with flexible, free-standing electronics and a 3D nanocomposite scaffold. The patch recorded the cellular electrical activities and the on-demand provision of electrical stimulation to synchronize cell contraction [112]. The researchers also showed that electroactive polymers containing biological factors could be deposited on designated electrodes in the patch for the release of drug on demand [112].
High-density lipoprotein-like nanoparticles have also been applied as a theranostic strategy for CAD. Recently, a study developed high-density lipoprotein-like magnetic nanostructures (HDL-MNS), synthesized by coating phospholipids and apoA1 onto magnetite nanostructures as a theranostic strategy for atherosclerosis [113]. HDL-MNS showed contrast by MRI that was five times higher than that of commercially available contrast agents (e.g., Ferumoxytol). Internalization of HDL-MNS by macrophages was confirmed and successfully imaged by MRI. Also, the HDL-MNS particles showed the ability to induce cholesterol efflux from macrophages comparable to natural HDL, thus providing a pathway to prevent and treat CAD via reverse cholesterol transport [113]. Recently, antioxidant nanoparticles and targeting of oxidative stress along with diagnostic nanoparticles as theranostic nanosystems for CVD have generated considerable interest [114,115].
The above-described studies showed evolution of the nanoparticles to theranostic tools with great potential for the diagnosis and treatment of CVD, specifically atherosclerosis and MI. A more general view of the evolution of the nanotechnology applied to CVD is shown in Figure 10. This tendency chart was made from the ISI publications reported on the Web of Knowledge database for the topics: i) Nanoparticles for CVD, ii) Nanoparticles for CVD diagnosis, iii) Nanoparticles for CVD therapy, and iv) Nanoparticles for CVD theranostics.
As shown in Figure 10, studies focused on nanotechnology for CVD showed an impressive increase since 2005. These studies were related to a greater number of publications on nanosystems for imaging and therapy of CVD. Before 2004, fewer than ten articles were published each year on these topics. This increase was initially due to the use of nanoparticles as contrast agents for cardiovascular imaging. After 2010, different approaches of nanoparticles for therapy showed a higher rate of increase in publications, surpassing the imaging applications. Publications using the concept of “theranostic” or “theragnosis” appeared in 2006, and to date, about 101 articles have been published. Before 2012, fewer than ten papers were published per year and after 2013, between 13 and 17 articles per year have been published, showing a tendency of slow growth of this topic. This analysis was performed only with articles that used the concept “theranostic” to describe the multifunctional nanosystems. This analysis shows the ongoing application of theranostic strategies to CVD, with is in contrast to theranostic strategies for cancer, which registered around 2,400 articles to date. This difference is related to the much earlier use of nanoparticles for cancer ablation and drug delivery due to their high accumulation in tumors. In recent years, nanosystems used as vehicles for drugs, contrast agents for imaging, and novel biomolecules for targeting are advancing the use of theranostic strategies for CVD.
Evolution of the number of ISI publications on nanoparticles for CVD, CVD therapy, diagnosis and theranostics.
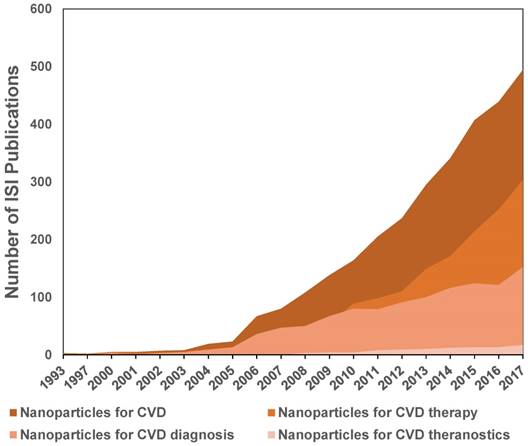
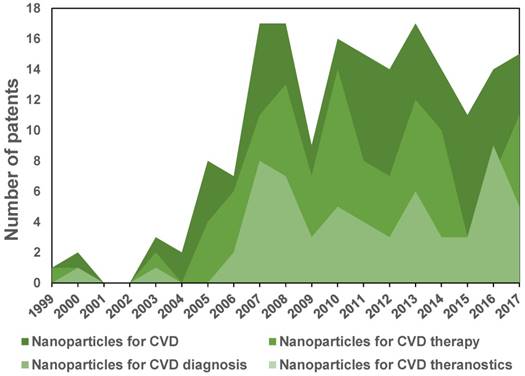
Figure 11 shows a tendency chart with the number of patents reported in the database of the World Intellectual Property Organization (WIPO). Patents related to nanosystems applied to CVD appeared around the 00´s with the first publications on iron oxide nanoparticles for MRI. After 2005, there was an increase of 10-20 applications per year with a tendency of a larger number of patents related to therapy than diagnosis. To date (2018), patents using the concept “theranostic” for cardiovascular applications do not exist in the WIPO database, whereas about 15 cancer patents were found, showing more significant activity in this field.
Toxicology of nanomaterials for potential theranostic applications
Despite the great potential of nanomaterials for theranostic applications [116], it is important to consider their potential toxic effects. Although the nanotoxicological aspects of nanomaterials are beyond the scope of this review, it is necessary to briefly consider the potential adverse effects of nanoparticles with theranostic applications. For more details on this topic, we refer readers to a recently published review [117].
Nanoparticles can generate toxicity by several mechanisms including ROS production, DNA injury, protein misfolding, membrane perturbation and direct physical damage. Almost all nanoparticles can generate ROS [117], while metal or metal oxide nanoparticles, such as titanium oxide nanoparticles, are more likely to induce oxidative stress, which is evident from the increase in lipid peroxidation levels in the liver, kidneys, and spleen [118]. On the other hand, cerium oxide nanoparticles reduced cell viability, increased ROS levels, lipid peroxidation and cellular membrane damage in a dosage-dependent manner [119,120]. ROS production-related oxidative damage to mitochondrial function was observed for silver nanoparticles [121]. Also, gold nanoparticles caused a down-regulation of antioxidant enzymes favoring oxidative stress [122].
Nanoparticles can also produce an inflammatory response. Numerous studies reported that nanoparticles could increase levels of several inflammatory cytokines and expression of relevant mRNAs. For instance, silica nanoparticles have been shown to cause a significant increase in the release of several pro-inflammatory cytokines [123] and carbon nanotubes could cause lesions in lung epithelia in a dose-dependent manner and persistent interstitial inflammation on chronic exposure [124]. Amphiphilic polymeric micelles, ((poly(ethylene glycol)-polyglycerol- poly(ε-caprolactone) (PEG-PG-PCL), poly(ethyl ethylene phosphate)-co-poly(ε-caprolactone) (PEEP-PCL), poly(ethylene glycol)-poly(ε-caprolactone) (PEG-PCL), and poly(ethylene glycol)-distearoyl-sn-glycero-phosphoethanolamine (PEG-DSPE)), induced changes in cell size at high concentrations and augmented pro-inflammatory molecules as a consequence of the increased ROS level, but no evidence of a toxic effect on cellular membranes was described [125].
Evolution of the number of patents on nanoparticles for CVD, CVD therapy and diagnosis. No patents were found for CVD theranostics up to 2016.
Nanoparticles can affect vascular hemostasis and blood platelet function. For example, nanodiamonds stimulated platelet aggregation and increased thrombosis in vitro and in vivo [126,127]. Other inorganic nanomaterials, such as silica nanoparticles [128], gold nanoparticles, and quantum dots may also stimulate platelet activation [129]. Zhu et al. reported that iron oxide NPs may increase microvascular permeability and cell lysis in lung epithelia and disturb blood coagulation parameters significantly [130].
The physicochemical properties of nanoparticles such as the aggregation state, charge, size, and surface properties can play an important role in toxicity, as was observed for carbon nanoparticles [131]. For example, smaller size nanoparticles commonly result in higher tissue distribution and commonly cause overt adverse effects compared to larger size nanoparticles such as silver and gold nanoparticles [132-134]. Certain coating materials like PEG and peptides could significantly reduce the potential toxicity of gold nanoparticles on cell viability [135]. Similarly, the functionalization of quantum dots with antibodies decreased the toxicity of these nanoparticles [136].
It is clear that there are potential risks involved in the use of nanomaterials. We summarized some of these adverse effects and some strategies to reduce these effects. Given the intrinsic properties of nanomaterials, their safe clinical application would be different from the classical paradigm established for conventional drugs. In this regard, the FDA and the EU agency have already set up robust schemes for the approval of new nanopharmaceuticals [137].
Future perspectives and clinical challenges
Nanomedicine, the medical application of nanotechnology, is a promising field of study primarily due to the unique properties obtained at the nanometer size. Research publications, clinical trials, and FDA-approved therapeutics and nanoparticle-containing imaging agents have been increasing in the last decades, and this trend is expected to continue. Most of these nanomedicine-based drugs and imaging agents that are FDA approved or in clinical trials entail passive targeting via the EPR effect [23, 140]. However, in recent years, the trend is to create more complex nanosystems with new materials incorporating active targeting molecules and the ability to modulate the effect in a spatial and temporal manner, as displayed schematically in Figure 12.
These challenges, in the context of CVD, necessitate precision drug delivery and early detection of atherosclerotic plaques. For this purpose, the identification of target molecules such as αvβ3-integrins (angiogenesis marker) and inflammation markers such as VCAM-1, ICAM-1 and E-selectin will be useful for active targeting of the atherosclerotic plaques [28,32,33]. For more details about active targeting related to inflammation markers and nanosystems, readers are referred to recent reviews covering these topics [139-141].
The important aspects for the development of the next generation of diagnostic nanodevices for medical applications include a) identification of new markers for designing active targeting and dual targeting approaches, b) use of different materials such as thermal-, optical-, or pH-sensitive polymers or lipids to improve the delivery, and c) taking advantage of some physical properties, such as plasmonic effect, to create hyperthermia. There are studies of new kinds of high-density lipoprotein (HDL)-like nanoparticles that are completely biocompatible and possess a natural affinity for the macrophages of atherosclerotic plaques [30]. These protein-based nanoparticles are good candidates to engineer nanosystems and by modification of their surfaces is possible to improve the vectorization. Furthermore, the use of exosomes appears to be an exciting approach to target organs and cells for nanotheranostic applications. Also, inorganic nanoparticles such as gold, magnetic nanoparticles, carbon nanotubes and lanthanides, continue to be important players for theranostic strategies in CVD.
Scheme of theranostic nanosystems used for CVD: (A) Scaffolds/tissue engineering, (B) inorganic nanoparticles/polymer coatings, (C) polymer nanoparticles/micelles, (D) lipid nanoparticles/vesicles.
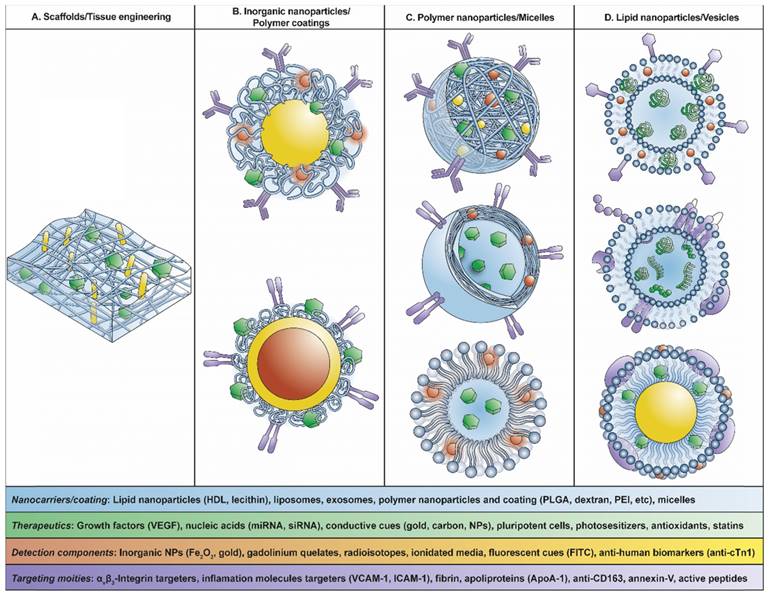
The combination of these nanostructures with biomolecules will lead to the evolution of nanodevices generating theranostic nanosystems with diagnosis and treatment in the same device. These nanosystems will be helpful in detecting the presence of atherosclerotic plaques in asymptomatic patients, which is the major challenge in CVD considering that early detection is fundamental to the outcome of patients with MI.
Despite the benefits and potential of the evolving theranostic nanosystems, their complexity presents a significant challenge to meet the FDA standards and achieve translation to clinical application. The FDA's draft guidance indicates that an exhaustive characterization of the nanomaterial is required, including physical and chemical characterization and pharmacological parameters [142]. Several aspects related to the clinical translation of nanomedicines need to be addressed before their effective and safe use in human patients. For instance, it is essential to ensure that the efficacy and safety results obtained with laboratory equipment are effectively the same during clinical analysis, as the techniques used in the laboratory are not directly applicable to the clinic. Furthermore, the behavior of nanosystems in the dynamic physiological and pathological alterations in vivo is not well defined. Survivability of nanoparticles in plasma and accumulation in non-targeted organs and tissues need to be further investigated as well as biocompatibility and biodegradability must be systematically established [143].
Another significant issue is the manufacturing process of nanomedicines, which is far more complicated than the current biopharmaceutical manufacturing. It is challenging to determine a scalable manufacturing system for nanomedicines and to standardize drug quality and functionality tests for clinical use. It is also not trivial to establish good manufacturing practices (GMP). To accomplish these objectives, it is necessary to increase the interaction between small laboratories and the pharmaceutical industry [144].
Conclusions
Nanotechnology has been called the industrial revolution of the 21st century, and its medical application, nanomedicine, has had promising advances such as drug delivery systems and imaging, among many others. Since the first nanoformulation for imaging associated to CVD at the end of the 80´s, development of theranostic strategies for CVD has attracted much attention-stimulating research with a potential to reach the clinic. The use of antibodies and peptides that recognize markers associated with CVD have allowed the development of a new generation of more selective nanosystems. This trend suggests that in the near future nanomedicine will be extensively used in the clinic, improving the quality of life of the population.
Abbreviations
4-AN: 4-amino-1,8-napthalimide; AnxCLIO‑Cy5.5: annexin V-cross-linked iron oxide- cyanine5.5); ApoA-1: apolipoprotein A1; ApoA-2: apolipoprotein A2; ApoE: apolipoproteína E; BMMSC: bone marrow-derived mesenchymal stem cells; CAD: coronary artery diseases; CCLs: cationic lipoparticles; CCR2: chemokine receptor; CD163: cluster of differentiation 163; CD31: cluster of differentiation 31; CD68: cluster of differentiation 68; CD68: cluster of differentiation 68; CDC: cardiosphere-derived cells; Ce6: chlorine6; CeO2: cerium oxide; CLIO-Cy5.5: cross-linked iron oxide-cyanine5.5; CLIO-VT750: cross-linked iron oxide-VT750; CM: confocal microscopy; CNT: carbon nanotubes; CPC: cardiac progenitor cells; CT: X-ray computed tomography; cTnI: cardiac troponin I; cTnT: cardiac troponin T; CVD: cardiovascular diseases; Cy5.5: cyanine5.5; Cy7: cyanine7; DAPI: 4′,6-diamidino-2-phenylindole; DNA: deoxyribonucleic acid; DSPC: 1,2-distearoyl-sn-glycero-3-phosphorylcholine; DSPE: 1,2-distearoyl-sn-glycero-3-phosphoethano-lamine; EF%: ejection fraction; ELISA: enzyme-linked immunosorbent assays; EPR: enhanced permeability and retention; ER: endoplasmic reticulum; FAM: carboxyfluorescein; FDA: U.S Food and Drug Administration; Fe3O4: magnetite; fGO: functionalized with graphene oxide; FITC: fluorescein isotiocyanate; FMT: multichannel fluorescent molecular tomography; FTIR: Fourier transform infrared spectroscopy; Gd-DTPA-DMPE: gadolinium chelated 1,2‑dimyristoyl-sn-glycero-3-phosphoethanolamine‑N‑diethylenetriaminepenta acetic acid; GelMA: methacrylated gelatin; GMP: good manufacturing practices; GNP: gold nanoparticles; GNR: gold nanorods; GNS: gold nanospheres; H2O2: hydrogen peroxide; HA: hyaluronic acid; HDL: high-density lipoprotein; HGF: hepatocyte growth factor; HMON: hollow mesoporous organosilica nanoparticles; HPOX: polyoxalate copolymer nanoparticles; Hsp70: 70-kDa heat shock protein; ICAM-1: intercellular adhesion molecule-1; IR: ischemia-reperfusion; LiLa: hybrid lipid-latex; LPS: lipopolysaccharide; Luc: luciferase; Met AP2: methionine aminopeptidase 2; MI: myocardial infarction; MNS: magnetic nanostructures; MPS: mononuclear phagocytic system; MRA: magnetic resonance angiography; MRI: magnetic resonance imaging; MSC: mesenchymal stem cells; MTX: mitoxantrone; nBG: bioactive glass nanoparticles; NIH: National Institutes of Health; Nkx2.5: cardiac homeobox protein; PE: phosphatidyl-ethanolamine; PEEP-PCL: poly(ethyl ethylene phosphate)-co-poly(ε-caprolactone); PEG: polyethyleneglycol; PEG-DSPE: poly(ethylene glycol)-distearoyl-sn-glycero-phosphoethanolamine; PEG-PCL: poly(ethylene glycol)-poly(ε-caprolactone); PEG-PG-PCL: poly(ethylene glycol)-polyglycerol- poly(ε-caprolactone); PEI: polyethyleneimine; PEI-DA: polyethyleneimine modified with deoxycholic acid; PET-CT: positron-emission tomography with X-ray computed tomography; PET-MRI: positron-emission tomography with magnetic resonance imaging; PLGA: poly(lactic-co-glycolic acid); PLN: platelet-like nanoparticles; R1: spin-lattice relaxation rate; R2: spin-spin relaxation rate; RES: reticuloendothelial system; rHDL: reconstituted high density lipoprotein nanoparticles; RNA: ribonucleic acid; ROI: region‑of‑interest; ROS: reactive oxygen species; SERS: surface-enhanced raman scattering; SHPC: stearoyl-hydroxy phosphatidylcholine; siRNA: small interfering ribonucleic acid; SPION: superparamagnetic iron oxide nanoparticles; SV40: simian virus 40; SWNT: single wall carbon nanotubes; TEM: transmission electron mycroscopy; TPC: 5-(4-carboxyphenyl)-10,15,20-triphenyl-2,3-dihydroxychlorine; USPIO: ultrasmall superparamagnetic iron oxide; VCAM-1: vascular cell adhesion molecule-1; VEGF: vascular endothelial growth factor; WIPO: World Intellectual Property Organization.
Acknowledgements
This work was supported by Comisión Nacional de Investigación Científica y Tecnológica (CONICYT), Chile, Fondo de Financiamiento Centros de Excelencia en Investigación en Áreas Prioritarias (FONDAP) grant 15130011 (to. S.L., M.K., P.F.C., H.E.V. F.M.Z and J.O.M) and Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) grants 1170929 (to M.K.) and 1181689 (to J.O.M). J.B. was supported by post-doctoral FONDECYT grants 3160323. M.N-M and I.G-C are recipients of a Ph.D. fellowship from CONICYT. EA acknowledges Proyecto Nucleo DI-36-18/N.
Competing Interests
The authors have declared that no competing interest exists.
References
1. World Health Organization. Media Centre: Cardiovascular disease. World Health Organization. 2017. Revised 1 May 2018. http://www.who.int/mediacentre/factsheets/fs317/en/
2. EHN. European cardiovascular disease statistics. Brussels: European Heart Network. 2017. Revised 1 May 2018. http://www.ehnheart.org/cvd-statistics.html
3. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R. et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146-e603
4. Cormode DP, Roessl E, Thran A, Skajaa T, Gordon RE, Schlomka J-P. et al. Atherosclerotic plaque composition: analysis with multicolor CT and targeted gold nanoparticles. Radiology. 2010;256:774-82
5. Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R. et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;63:2935-59
6. Dweck MR, Doris MK, Motwani M, Adamson PD, Slomka P, Dey D. et al. Imaging of coronary atherosclerosis—evolution towards new treatment strategies. Nature Reviews Cardiology. 2016;13:533-48
7. Kullo IJ, Cooper LT. Early identification of cardiovascular risk using genomics and proteomics. Nature Reviews Cardiology. 2010;7:309-17
8. Fuster V, Lois F, Franco M. Early identification of atherosclerotic disease by noninvasive imaging. Nature Reviews Cardiology. 2010;7:327-33
9. Topol EJ, Nissen SE. Our preoccupation with coronary luminology: the dissociation between clinical and angiographic findings in ischemic heart disease. Circulation. 1995;92:2333-42
10. Alderman EL, Corley SD, Fisher LD, Chaitman BR, Faxon DP, Foster ED. et al. Five-year angiographic follow-up of factors associated with progression of coronary artery disease in the Coronary Artery Surgery Study (CASS). Journal of the American College of Cardiology. 1993;22:1141-54
11. Husted SE, Ohman EM. Pharmacological and emerging therapies in the treatment of chronic angina. The Lancet. 2015;386:691-701
12. Authors/Task Force M, Hamm CW, Bassand J-P, Agewall S, Bax J, Boersma E. et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). European heart journal. 2011;32:2999-3054
13. Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. The Journal of clinical investigation. 2013;123:92-100
14. Bulluck H, Yellon DM, Hausenloy DJ. Reducing myocardial infarct size: challenges and future opportunities. Heart. 2015 heartjnl-2015-307855
15. Mukherjee B, Dey NS, Maji R, Bhowmik P, Das PJ, Paul P. Chapter 16: Current status and future scope for nanomaterials in drug delivery. Application of Nanotechnology in Drug Delivery: IntechOpen. 2014
16. Bamrungsap S, Zhao Z, Chen T, Wang L, Li C, Fu T. et al. Nanotechnology in therapeutics: a focus on nanoparticles as a drug delivery system. Nanomedicine. 2012;7:1253-71
17. Nune SK, Gunda P, Thallapally PK, Lin Y-Y, Laird Forrest M, Berkland CJ. Nanoparticles for biomedical imaging. Expert opinion on drug delivery. 2009;6:1175-94
18. Wilczewska AZ, Niemirowicz K, Markiewicz KH, Car H. Nanoparticles as drug delivery systems. Pharmacological reports. 2012;64:1020-37
19. Fan Z, Fu PP, Yu H, Ray PC. Theranostic nanomedicine for cancer detection and treatment. Journal of food and drug analysis. 2014;22:3-17
20. Wang Y-J, Larsson M, Huang W-T, Chiou S-H, Nicholls SJ, Chao J-I. et al. The use of polymer-based nanoparticles and nanostructured materials in treatment and diagnosis of cardiovascular diseases: Recent advances and emerging designs. Progress in Polymer Science. 2016;57:153-78
21. Caldorera-Moore ME, Liechty WB, Peppas NA. Responsive theranostic systems: integration of diagnostic imaging agents and responsive controlled release drug delivery carriers. Accounts of chemical research. 2011;44:1061-70
22. Mura S, Couvreur P. Nanotheranostics for personalized medicine. Advanced drug delivery reviews. 2012;64:1394-416
23. Anselmo AC, Mitragotri S. Nanoparticles in the clinic. Bioengineering & translational medicine. 2016;1:10-29
24. Weissleder R, Elizondo G, Wittenberg J, Rabito CA, Bengele HH, Josephson L. Ultrasmall superparamagnetic iron oxide: characterization of a new class of contrast agents for MR imaging. Radiology. 1990;175:489-93
25. Weissleder R, Lee AS, Fischman AJ, Reimer P, Shen T, Wilkinson R. et al. Polyclonal human immunoglobulin G labeled with polymeric iron oxide: antibody MR imaging. Radiology. 1991;181:245-9
26. Lanza GM, Lorenz CH, Fischer SE, Scott MJ, Cacheris WP, Kaufmann RJ. et al. Enhanced detection of thrombi with a novel fibrin-targeted magnetic resonance imaging agent. Academic radiology. 1998;5:S173-S6
27. Yu X, Song SK, Chen J, Scott MJ, Fuhrhop RJ, Hall CS. et al. High-resolution MRI characterization of human thrombus using a novel fibrin-targeted paramagnetic nanoparticle contrast agent. Magnetic resonance in medicine. 2000;44:867-72
28. Winter PM, Morawski AM, Caruthers SD, Fuhrhop RW, Zhang H, Williams TA. et al. Molecular imaging of angiogenesis in early-stage atherosclerosis with αvβ3-integrin-targeted nanoparticles. Circulation. 2003;108:2270-4
29. Flacke S, Fischer S, Scott MJ, Fuhrhop RJ, Allen JS, McLean M. et al. Novel MRI contrast agent for molecular imaging of fibrin implications for detecting vulnerable plaques. Circulation. 2001;104:1280-5
30. Frias JC, Williams KJ, Fisher EA, Fayad ZA. Recombinant HDL-like nanoparticles: A specific contrast agent for MRI of atherosclerotic plaques. J Am Chem Soc. 2004;126:16316-7
31. Hyafil F, Cornily JC, Feig JE, Gordon R, Vucic E, Amirbekian V. et al. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat Med. 2007;13:636-41
32. Kim K, Huang SW, Ashkenazi S, O'Donnell M, Agarwal A, Kotov NA. et al. Photoacoustic imaging of early inflammatory response using gold nanorods. Appl Phys Lett. 2007:90
33. Kelly KA, Allport JR, Tsourkas A, Shinde-Patil VR, Josephson L, Weissleder R. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ Res. 2005;96:327-36
34. Cormode DP, Skajaa T, van Schooneveld MM, Koole R, Jarzyna P, Lobatto ME. et al. Nanocrystal core high-density lipoproteins: A multimodal molecular imaging contrast agent platform. Nano Lett. 2008;8:3715-23
35. Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E. et al. Nanoparticle PET-CT Imaging of Macrophages in Inflammatory Atherosclerosis. Circulation. 2008;117:379-87
36. Uwatoku T, Shimokawa H, Abe K, Matsumoto Y, Hattori T, Oi K. et al. Application of nanoparticle technology for the prevention of restenosis after balloon injury in rats. Circ Res. 2003;92:62-69
37. Nakano K, Egashira K, Masuda S, Funakoshi K, Zhao G, Kimura S. et al. Formulation of nanoparticle-eluting stents by a cationic electrodeposition coating technology. JACC Cardiovasc Interv. 2009;2:277-83
38. Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G. et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005-10
39. Duivenvoorden R, Tang J, Cormode DP, Mieszawska AJ, Izquierdo-Garcia D, Ozcan C. et al. A Statin-Loaded Reconstituted High-Density Lipoprotein Nanoparticle Inhibits Atherosclerotic Plaque Inflammation. Nat Commun. 2014;5:3065
40. Sanchez-Gaytan BL, Fay F, Lobatto ME, Tang J, Ouimet M, Kim Y. et al. HDL-mimetic PLGA nanoparticle to target atherosclerosis plaque macrophages. Bioconjug Chem. 2015;26:443-51
41. Wang Y-XJ. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant Imaging Med Surg. 2011;1:35-44
42. Anzai Y, Prince MR, Chenevert TL, Maki JH, Londy F, London M. et al. MR angiography with an ultrasmall superparamagnetic iron oxide blood pool agent. J Magn Reson Imaging. 1997;7:209-14
43. Ruehm SG, Corot C, Vogt P, Kolb S, Debatin JF. Magnetic resonance imaging of atherosclerotic plaque with ultrasmall superparamagnetic particles of iron oxide in hyperlipidemic rabbits. Circulation. 2001;103:415-22
44. Kooi ME. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107:2453-8
45. Morishige K, Kacher DF, Libby P, Josephson L, Ganz P, Weissleder R. et al. High-resolution magnetic resonance imaging enhanced with superparamagnetic nanoparticles measures macrophage burden in atherosclerosis. Circulation. 2010;122:1707-15
46. Tarin C, Carril M, Martin-Ventura JL, Markuerkiaga I, Padro D, Llamas-Granda P. et al. Targeted gold-coated iron oxide nanoparticles for CD163 detection in atherosclerosis by MRI. Sci Rep. 2015;5:17135
47. Kim D, Park S, Lee JH, Jeong YY, Jon S. Antibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo X-ray computed tomography imaging. J Am Chem Soc. 2007;129:12585
48. Li W, Chen X. Gold nanoparticles for photoacoustic imaging. Nanomedicine (Lond). 2015;10:299-320
49. Wang B, Yantsen E, Larson T, Karpiouk AB, Sethuraman S, Su JL. et al. Plasmonic intravascular photoacoustic imaging for detection of macrophages in atherosclerotic plaques. Nano Lett. 2008;9:2212-7
50. Pissuwan D, Hattori Y. Detection of adhesion molecules on inflamed macrophages at early-stage using SERS probe gold nanorods. Nano-Micro Lett. 2017;9:8
51. Anselmo AC, Modery-Pawlowski CL, Menegatti S, Kumar S, Vogus DR, Tian LL. et al. Platelet-like nanoparticles (PLNs): engineering shape, flexibility and surface chemistry of nanocarriers to target vascular injuries. ACS Nano. 2014;8:11243-53
52. Kheirolomoom A, Kim CW, Seo JW, Kumar S, Son DJ, Gagnon MKJ. et al. Multifunctional nanoparticles facilitate molecular targeting and miRNA delivery to inhibit atherosclerosis in ApoE-/- mice. ACS Nano. 2015;9:8885-97
53. Sun T, Simmons R, Huo D, Pang B, Zhao X, Kim CW. et al. Targeted Delivery of Anti-miR-712 by VCAM1-Binding Au Nanospheres for Atherosclerosis Therapy. ChemNanoMat. 2016;2:400-6
54. Rozenman Y, Zou X, Neuringer L, Kantor HL. The use of superparamagnetic ferrite particles in the detection of myocardial reperfusion. Proceedings of the International Society for Magnetic Resonance in Medicine Amsterdam. 1989 p. 673
55. Sosnovik DE, Schellenberger EA, Nahrendorf M, Novikov MS, Matsui T, Dai G. et al. Magnetic resonance imaging of cardiomyocyte apoptosis with a novel magneto-optical nanoparticle. Magn Reson Med. 2005;54:718-24
56. Nahrendorf M, Sosnovik DE, Waterman P, Swirski FK, Pande AN, Aikawa E. et al. Dual channel optical tomographic imaging of leukocyte recruitment and protease activity in the healing myocardial infarct. Circ Res. 2007;100:1218-25
57. Lukyanov AN, Hartner WC, Torchilin VP. Increased accumulation of PEG-PE micelles in the area of experimental myocardial infarction in rabbits. J Control Release. 2004;94:187-93
58. Majmudar MD, Keliher EJ, Heidt T, Leuschner F, Truelove J, Sena BF. et al. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation. 2013;127:2038-46
59. Kim D, Hong J, Moon HH, Nam HY, Mok H, Jeong JH. et al. Anti-apoptotic cardioprotective effects of SHP-1 gene silencing against ischemia-reperfusion injury: Use of deoxycholic acid-modified low molecular weight polyethyleneimine as a cardiac siRNA-carrier. J Control Release. 2013;168:125-34
60. Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM. et al. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function aftermyocardial infarction. Cardiovasc Res. 2014;103:530-41
61. Ichimura K, Matoba T, Nakano K, Tokutome M, Honda K, Koga J-I. et al. A Translational study of a new therapeutic approach for acute myocardial infarction: nanoparticle-mediated delivery of pitavastatin into reperfused myocardium reduces ischemia-reperfusion injury in a preclinical porcine model. PLoS One. 2016;11:e0162425
62. Guo ZR, Gu CR, Fan X, Bian ZP, Wu HF, Yang D. et al. Fabrication of anti-human cardiac troponin I immunogold nanorods for sensing acute myocardial damage. Nanoscale Res Lett. 2009;4:1428-33
63. Yang X, Zhao Y, Sun L, Qi H, Gao Q, Zhang C. Electrogenerated chemiluminescence biosensor array for the detection of multiple AMI biomarkers. Sensors Actuators, B Chem. 2018;257:60-7
64. Li Z, Zhang J, Li Y, Zhao S, Zhang P, Zhang Y. et al. Carbon dots based photoelectrochemical sensors for ultrasensitive detection of glutathione and its applications in probing of myocardial infarction. Biosens Bioelectron. 2018;99:251-8
65. Tang M, Zhou Z, Shangguan L, Zhao F, Liu S. Electrochemiluminescent detection of cardiac troponin I by using soybean peroxidase labeled-antibody as signal amplifier. Talanta. 2018;180:47-53
66. Oh KS, Song JY, Yoon SJ, Park Y, Kim D, Yuk SH. Temperature-induced gel formation of core/shell nanoparticles for the regeneration of ischemic heart. J Control Release. 2010;146:207-11
67. Dvir T, Timko BP, Brigham MD, Naik SR, Karajanagi SS, Levy O. et al. Nanowired three-dimensional cardiac patches. Nat Nanotechnol. 2011;6:720-5
68. You JO, Rafat M, Ye GJC, Auguste DT. Nanoengineering the heart: conductive scaffolds enhance connexin 43 expression. Nano Lett. 2011;11:3643-8
69. Mooney E, Mackle JN, Blond DJP, O'Cearbhaill E, Shaw G, Blau WJ. et al. The electrical stimulation of carbon nanotubes to provide a cardiomimetic cue to MSCs. Biomaterials. 2012;33:6132-9
70. Kim D-H, Kshitiz, Smith RR, Kim P, Ahn EH, Kim H-N. et al. Nanopatterned cardiac cell patches promote stem cell niche formation and myocardial regeneration. Integr Biol. 2012;4:1019-33
71. Shevach M, Fleischer S, Shapira A, Dvir T. Gold Nanoparticle-Decellularized Matrix Hybrids for Cardiac Tissue Engineering. Nano Lett. 2014;14:5792-6
72. Paul A, Hasan A, Kindi H Al, Gaharwar AK, Rao VTS, Nikkhah M. et al. Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair. ACS Nano. 2014;8:8050-62
73. Shevtsov MA, Nikolaev BP, Ryzhov VA, Yakovleva LY, Dobrodumov A V, Marchenko YY. et al. Detection of experimental myocardium infarction in rats by MRI using heat shock protein 70 conjugated superparamagnetic iron oxide nanoparticle. Nanomedicine Nanotechnology, Biol Med. 2016;12:611-21
74. Ashton JR, West JL, Badea CT. In vivo small animal micro-CT using nanoparticle contrast agents. Front Pharmacol. 2015;6:1-22
75. Chapon C, Franconi F, Lemaire L, Marescaux L, Legras P, Saint-André J-P. et al. High field magnetic resonance imaging evaluation of superparamagnetic iron oxide nanoparticles in a permanent rat myocardial infarction. Invest Radiol. 2003;38:141-6
76. Kraitchman DL, Heldman AW, Atalar E, Amado LC, Martin BJ, Pittenger MF. et al. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107:2290-3
77. Amsalem Y, Mardor Y, Feinberg MS, Landa N, Miller L, Daniels D. et al. Iron-oxide labeling and outcome of transplanted mesenchymal stem cells in the infarcted myocardium. Circulation. 2007;116:38-46
78. Alam SR, Shah AS V, Richards J, Lang NN, Barnes G, Joshi N. et al. Ultrasmall superparamagnetic particles of iron oxide in patients with acute myocardial infarction early clinical experience. Circ Cardiovasc Imaging. 2012;5:559-65
79. Yilmaz A, Dengler MA, Van Der Kuip H, Yildiz H, R??sch S, Klumpp S. et al. Imaging of myocardial infarction using ultrasmall superparamagnetic iron oxide nanoparticles: A human study using a multi-parametric cardiovascular magnetic resonance imaging approach. Eur Heart J. 2013;34:462-75
80. Danila D, Johnson E, Kee P. CT imaging of myocardial scars with collagen-targeting gold nanoparticles. Nanomedicine Nanotechnology, Biol Med. 2013;9:1067-76
81. Sawall S, Franke D, Kirchherr A, Beckendorf J, Kuntz J, Maier J. et al. In Vivo Quantification of Myocardial Infarction in Mice Using Micro-CT and a Novel Blood Pool Agent. Contrast Media Mol I. 2017 Article ID 2617047
82. Cormode DP, Si-Mohamed S, Bar-Ness D, Sigovan M, Naha PC, Balegamire J. et al. Multicolor spectral photon-counting computed tomography: In vivo dual contrast imaging with a high count rate scanner. Sci Rep. 2017;7:1-11
83. Niu J, Azfer A, Rogers LM, Wang X, Kolattukudy PE. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc Res. 2007;73:549-59
84. Davidson SM, Takov K, Yellon DM. Exosomes and Cardiovascular Protection. Cardiovasc drugs Ther. 2016;31:77-86
85. Ailawadi S, Wang X, Gu H, Fan G-C. Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochim Biophys Acta-Mol Basis Dis. 2015;1852:1-11
86. Fleury A, Martinez MC, Le Lay S. Extracellular vesicles as therapeutic tools in cardiovascular diseases. Front Immunol. 2014;5:1-8
87. Giricz Z, Varga Z V, Baranyai T, Sipos P, Pálóczi K, Kittel Á. et al. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J Mol Cell Cardiol. 2014;68:75-8
88. Zhu K, Wu M, Lai H, Guo C, Li J, Wang Y. et al. Nanoparticle-enhanced generation of gene-transfected mesenchymal stem cells for in vivo cardiac repair. Biomaterials. 2016;74:188-99
89. Webber MJ, Tongers J, Newcomb CJ, Marquardt K, Bauersachs J, Losordo DW. et al. Supramolecular nanostructures that mimic VEGF as a strategy for ischemic tissue repair. Proc Natl Acad Sci. 2011;108:13438-43
90. Barabadi Z, Azami M, Sharifi E, Karimi R, Lotfibakhshaiesh N, Roozafzoon R. et al. Fabrication of hydrogel-based nanocomposite scaffold containing bioactive glass nanoparticles for myocardial tissue engineering. Mater Sci Eng C. 2016;69:1137-46
91. Waters R, Pacelli S, Maloney R, Medhi I, Ahmed RPH, Paul A. Stem cell secretome-rich nanoclay hydrogel: a dual action therapy for cardiovascular regeneration. Nanoscale. 2016:7371-6
92. National Institute of Health (NHI). Theranostic Nanomedicine Section. 2017. Revised 15 May 2018. https://www.nibib.nih.gov/labs-at-nibib/theranostic-nanomedicine-section
93. Picard FJ, Bergeron MG. Rapid molecular theranostics in infectious diseases. Drug Discov Today. 2002;7:1092-101
94. McCarthy JR, Jaffer FA, Weissleder R. A macrophage-targeted theranostic nanoparticle for biomedical applications. Small. 2006;2:983-7
95. Winter PM, Neubauer AM, Caruthers SD, Harris TD, Robertson JD, Williams TA. et al. Endothelial ανβ3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2103-9
96. Polyak B, Fishbein I, Chorny M, Alferiev I, Williams D, Yellen B. et al. High field gradient targeting of magnetic nanoparticle-loaded endothelial cells to the surfaces of steel stents. Proc Natl Acad Sci U S A. 2008;105:698-703
97. Ma LL, Feldman MD, Tam JM, Paranjape AS, Cheruku KK, Larson T a. et al. Small multifunctional nanoclusters (nanoroses) for targeted cellular imaging and therapy. ACS Nano. 2009;3:2686-96
98. McCarthy JR, Korngold E, Weissleder R, Jaffer FA. A light-activated theranostic nanoagent for targeted macrophage ablation in inflammatory atherosclerosis. Small. 2010;6:2041-9
99. Peters D, Kastantin M, Kotamraju VR, Karmali PP, Gujraty K, Tirrell M. et al. Targeting atherosclerosis by using modular, multifunctional micelles. Proc Natl Acad Sci U S A. 2009;106:9815-9
100. Hamzah J, Kotamraju VR, Seo JW, Agemy L, Fogal V, Mahakian LM. et al. Specific penetration and accumulation of a homing peptide within atherosclerotic plaques of apolipoprotein E-deficient mice. Proc Natl Acad Sci. 2011;108:7154-9
101. She ZG, Hamzah J, Kotamraju VR, Pang HB, Jansen S, Ruoslahti E. Plaque-penetrating peptide inhibits development of hypoxic atherosclerotic plaque. J Control Release. 2016;238:212-20
102. Kosuge H, Sherlock SP, Kitagawa T, Dash R, Robinson JT, Dai H. et al. Near-infrared imaging and photothermal ablation of vascular inflammation using single-walled carbon nanotubes. J Am Heart Assoc. 2012;1:1-10
103. Lee D, Bae S, Ke Q, Lee J, Song B, Karumanchi SA. et al. Hydrogen peroxide-responsive copolyoxalate nanoparticles for detection and therapy of ischemia-reperfusion injury. J Control Release. 2013;172:1102-10
104. Kim H, Kim Y, Kim IH, Kim K, Choi Y. ROS-Responsive activatable photosensitizing agent for imaging and photodynamic therapy of activated macrophages. Theranostics. 2014;4:1-11
105. Cheng K, Shen D, Hensley MT, Middleton R, Sun B, Liu W. et al. Magnetic antibody-linked nanomatchmakers for therapeutic cell targeting. Nat Commun. 2014;5:4880
106. Bagalkot V, Badgeley MA, Kampfrath T, Deiuliis JA, Rajagopalan S, Maiseyeu A. Hybrid nanoparticles improve targeting to inflammatory macrophages through phagocytic signals. J Control Release. 2015;217:243-55
107. Qin J, Peng Z, Li B, Ye K, Zhang Y, Yuan F. et al. Gold nanorods as a theranostic platform for in vitro and in vivo imaging and photothermal therapy of inflammatory macrophages. Nanoscale. 2015;7:13991-4001
108. Kharlamov AN, Tyurnina AE, Veselova VS, Kovtun OP, Shur VY, Gabinsky JL. Silica-gold nanoparticles for atheroprotective management of plaques: results of the NANOM-FIM trial. Nanoscale. 2015;7:8003-15
109. Sun X, Li W, Zhang X, Qi M, Zhang Z, Zhang X-E. et al. In vivo targeting and imaging of atherosclerosis using multifunctional virus-like particles of Simian Virus 40. Nano Lett. 2016;16:6164-71
110. Tian F, Conde J, Bao C, Chen Y, Curtin J, Cui D. Gold nanostars for efficient in vitro and in vivo real-time SERS detection and drug delivery via plasmonic-tunable Raman/FTIR imaging. Biomaterials. 2016;106:87-97
111. Cowan DB, Yao R, Akurathi V, Snay ER, Thedsanamoorthy JK, Zurakowski D. et al. Intracoronary delivery of mitochondria to the ischemic heart for cardioprotection. PLoS One. 2016;11:1-19
112. Feiner R, Engel L, Fleischer S, Malki M, Gal I, Shapira A. et al. Engineered hybrid cardiac patches with multifunctional electronics for online monitoring and regulation of tissue function. Nat Mater. 2016;15:1-8
113. Nandwana V, Ryoo S-R, Kanthala S, McMahon KM, Rink JS, Li Y. et al. High-density lipoprotein-like magnetic nanostructures (HDL-MNS): theranostic agents for cardiovascular disease. Chem Mater. 2017;29:2276-82
114. Kim KS, Song CG, Kang PM. Targeting Oxidative Stress Using Nanoparticles as a Theranostic Strategy for Cardiovascular Diseases. Antioxidants & redox signaling. 2018 doi: 10.1089/ars.2017.7428
115. Amani H, Habibey R, Hajmiresmail SJ, Latifi S, Pazoki-Toroudi H, Akhavan O. Antioxidant nanomaterials in advanced diagnoses and treatments of ischemia-reperfusion injuries. Journal of Materials Chemistry B. 2017;5:9452-9476
116. Wagner V, Dullaart A, Bock AK, Zweck A. The emerging nanomedicine landscape. Nat Biotechnol. 2006;24:1211-7
117. Wu T, Tang M. Review of the effects of manufactured nanoparticles on mammalian target organs. J Appl Toxicol. 2018;38:25-40
118. El-Shenawy NS, Al-Harbi MS, Al Hamayani FFE. Hormonal and organ-specific dysfunction induced by the interaction between titanium dioxide nanoparticles and salicylic acid in male mice. J Basic Clin Physiol Pharmacol. 2016;27:425-35
119. Kumari M, Singh SP, Chinde S, Rahman MF, Mahboob M, Grover P. Toxicity study of cerium oxide nanoparticles in human neuroblastoma cells. Int J Toxicol. 2014;33:86-97
120. Lin W, Huang YW, Zhou XD, Ma Y. Toxicity of cerium oxide nanoparticles in human lung cancer cells. Int J Toxicol. 2006;25:451-7
121. Teodoro JS, Silva R, Varela AT, Duarte FV, Rolo AP, Hussain S. et al. Low-dose, subchronic exposure to silver nanoparticles causes mitochondrial alterations in Sprague-Dawley rats. Nanomedicine. 2016;11:1359-75
122. Siddiqi NJ, Abdelhalim MAK, El-ansary AK, Alhomida AS, Ong WY. Identification of potential biomarkers of gold nanoparticle toxicity in rat brains. J Neuroinflammation. 2012;9:1-7
123. Zhao Y, Ye Y, Zhou X, Chen J, Jin Y, Hanson A. et al. Photosensitive fluorescent dye contributes to phototoxicity and inflammatory responses of dye-doped silica NPs in cells and mice. Theranostics. 2014;4:445-59
124. Fard JK, Jafari S, Eghbal MA. A review of molecular mechanisms involved in toxicity of nanoparticles. Adv Pharm Bull. 2015;5:447-54
125. Zhao B, Wang XQ, Wang XY, Zhang H, Dai WB, Wang J. et al. Nanotoxicity comparison of four amphiphilic polymeric micelles with similar hydrophilic or hydrophobic structure. Part Fibre Toxicol. 2013;10:1
126. Radomski A, Jurasz P, Alonso-Escolano D, Drews M, Morandi M, Malinski T. et al. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br J Pharmacol. 2005;146:882-93
127. Gaffney AM, Santos-Martinez MJ, Satti A, Major TC, Wynne KJ, Gun'ko YK. et al. Blood biocompatibility of surface-bound multi-walled carbon nanotubes. Nanomedicine Nanotechnology, Biol Med. 2015;11:39-46
128. Corbalan JJ, Medina C, Jacoby A. Amorphous silica nanoparticles aggregate human platelets: potential implications for vascular homeostasis. Int J Nanomedicine. 2012;7:631-9
129. Samuel SP, Santos-Martinez MJ, Medina C, Jain N, Radomski MW, Prina-Mello A. et al. CdTe quantum dots induce activation of human platelets: Implications for nanoparticle hemocompatibility. Int J Nanomedicine. 2015;10:2723-34
130. Zhu MT, Feng WY, Wang B, Wang TC, Gu YQ, Wang M. et al. Comparative study of pulmonary responses to nano- and submicron-sized ferric oxide in rats. Toxicology. 2008;247:102-11
131. Kreyling WG, Semmler-Behnke M, Seitz J, Scymczak W, Wenk A, Mayer P. et al. Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates fürom the lung of rats to the blood and secondary target organs. Inhal Toxicol. 2009;21:55-60
132. Recordati C, De Maglie M, Bianchessi S, Argentiere S, Cella C, Mattiello S. et al. Tissue distribution and acute toxicity of silver after single intravenous administration in mice: Nano-specific and size-dependent effects. Part Fibre Toxicol. 2016;13:1-17
133. Lasagna-Reeves C, Gonzalez-Romero D, Barria MA, Olmedo I, Clos A, Sadagopa Ramanujam VM. et al. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem Biophys Res Commun. 2010;393:649-55
134. Pan Y, Neuss S, Leifert A, Fischler M, Wen F, Simon U. et al. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3:1941-9
135. Ruff C, Hassan N, Morales-Zavala F, Steitz d EA, Kogan MJ, Simon U. CLPFFD-PEG Functionalized NIR-absorbing Hollow Gold Nanospheres and Gold Nanorods Inhibit ß-Amyloid Aggregation. J Mater Chem B. 2018;6:2432-43
136. Ma-Hock L, Brill S, Wohlleben W, Farias PMA, Chaves CR, Tenório DPLA. et al. Short term inhalation toxicity of a liquid aerosol of CdS/Cd(OH)2 core shell quantum dots in male Wistar rats. Toxicol Lett. 2012;208:115-24
137. Radomska A, Leszczyszyn J, Radomski M. The Nanopharmacology and Nanotoxicology of nanomaterials: new opportunities and challenges. Adv Clin Exp Med. 2016;25:151-62
138. Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33:2373-87
139. Myerson JW, Anselmo AC, Liu Y, Mitragotri S, Eckmann DM, Muzykantov VR. Non-affinity factors modulating vascular targeting of nano- and microcarriers. Adv Drug Deliv Rev. 2016;99:97-112
140. Muzykantov VR. Targeted drug delivery to endothelial adhesion molecules. ISRN Vasc Med. 2013 Article ID 916254: 27
141. Chacko AM, Hood ED, Zern BJ, Muzykantov VR. Targeted nanocarriers for imaging and therapy of vascular inflammation. Curr Opin Colloid Interface Sci. 2011;16:215-27
142. FDA-CDER. Drug products, including biological products, that contain nanomaterials - guidance for industry. U.S. Department of Health and Human Services Food and Drug Administration. 2017. Revised 25 May 2018. https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm
143. Bostan HB, Rezaee R, Valokala MG, Tsarouhas K, Golokhvast K, Tsatsakis AM. et al. Cardiotoxicity of nano-particles. Life Sci. 2016;165:91-9
144. Ragelle H, Danhier F, Préat V, Langer R, Anderson DG. Nanoparticle-based drug delivery systems: a commercial and regulatory outlook as the field matures. Expert Opin Drug Deliv. 2017;14:851-64
Author contact
![]() Corresponding authors: Sergio Lavandero (E-mail: slavandercl) & Marcelo J. Kogan (E-mail: mkoganuchile.cl). Advanced Center for Chronic Diseases (ACCDiS), Facultad Ciencias Químicas y Farmaceuticas, Universidad de Chile, Olivos 1007, Santiago 8380492, Chile.
Corresponding authors: Sergio Lavandero (E-mail: slavandercl) & Marcelo J. Kogan (E-mail: mkoganuchile.cl). Advanced Center for Chronic Diseases (ACCDiS), Facultad Ciencias Químicas y Farmaceuticas, Universidad de Chile, Olivos 1007, Santiago 8380492, Chile.
 Global reach, higher impact
Global reach, higher impact