13.3
Impact Factor
Theranostics 2019; 9(4):1001-1014. doi:10.7150/thno.30056 This issue Cite
Research Paper
Tumor-secreted dickkopf2 accelerates aerobic glycolysis and promotes angiogenesis in colorectal cancer
1. Department of Pathology, Nanfang Hospital, Southern Medical University, Guangzhou, China
2. Department of Pathology, School of Basic Medical Sciences, Southern Medical University, Guangzhou, China
3. Gastrointestinal Surgical Center, the First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
4. Department of Medical Oncology, Affiliated Tumor Hospital of Guangzhou Medical University, Guangzhou, China
5. Department of General Surgery, Zhujiang Hospital, Southern Medical University, Guangzhou, China
6. Department of General Surgery, Guangdong General Hospital, Guangdong Academy of Medical Science, Guangzhou, China.
7. Second Department of Hepatobiliary Surgery, Zhujiang Hospital, Southern Medical University, Guangzhou, China
* These authors are contributed equally to this work
Received 2018-9-18; Accepted 2019-1-14; Published 2019-1-30
Abstract
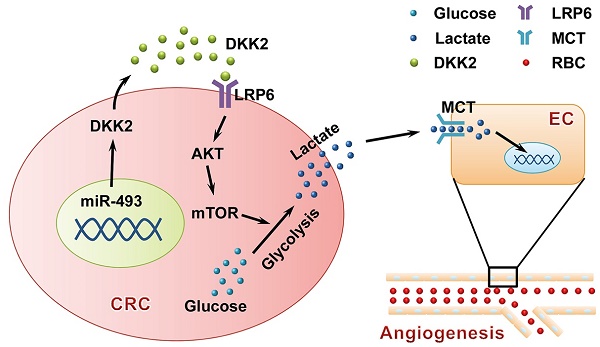
Angiogenesis is a fundamental process that involves in tumor progression and metastasis. Vascular endothelial growth factor (VEGF) family and their receptors are identified as the most prominent regulators of angiogenesis. However, the clinical efficacy of anti-VEGF/VEGFR therapy is not ideal, prompting the needs to further understand mechanisms behind tumor angiogenesis. Here, we found that Dickkopf associated protein 2 (DKK2), a secretory protein highly expressed in metastatic colorectal cancer tissues, could stimulate angiogenesis via a classic VEGF/VEGFR independent pathway.
Methods: DKK2 was screened out from microarray data analyzing gene expression profiles of eight pairs of non-metastatic and metastatic human colorectal cancer (CRC) tissues. Immunofluorescence histochemical staining (IHC) was used to detect the expression of DKK2 and angiogenesis in CRC tissues. Chicken chorioallantoic membrane (CAM) assay and Human umbilical vein endothelial cells (HUVEC) tubule formation assay was used for in vitro and in vivo angiogenesis study, respectively. Lactate and glucose concentration in the culture medium was measured by enzyme-linked immunosorbent assay (ELISA). Luciferase reporter assay was used to verify the interaction between miR-493-5p and the 3'UTR of DKK2.
Results: DKK2 could stimulate angiogenesis via accelerating the aerobic glycolysis of CRC cells, through which lactate is produced from glucose and accumulated in tumor microenvironment. Lactate functions as the final executor of DDK2 to stimulate tube formation of endothelial cells, and blockage of lactate secretion by lactate transporter (MCT) inhibitors dramatically neutralize the progression and metastasis of CRC both in vitro and in vivo. DKK2 could cooperate with lipoprotein receptor-related protein 6, which is required for glucose uptake, and activated the downstream mTOR signal pathway to accelerate lactate secretion. In addition, the expression of DKK2 is switched on via the demethylation of miR-493-5p, which allows the dissociated of miR-493-5p from the 3′-UTRs of DKK2 and initiates its stimulatory role on CRC progression in an autocrine or paracrine manner.
Conclusion: DKK2 promotes tumor metastasis and angiogenesis through a novel VEGF-independent, but energy metabolism related pathway. DKK2 might be a potential anti-angiogenic target in clinical treatment for the advanced CRC patients.
Keywords: Colorectal cancer, Vascular endothelial growth factor, Dickkopf 2, Aerobic Glycolysis, Angiogenesis
Introduction
Colorectal cancer (CRC) is the fourth most common malignances and the third leading cause of cancer deaths worldwide [1]. With the improvement of diagnostic and therapeutic strategies, the survival time of CRC patients has dramatically increased in recent years, but the mortality rate remains high [2]. Especially, advanced CRC patients still suffer from a poor 5-year survival rate lower than 10% [3]. Aberrant regulation of angiogenesis is widely believed to be one the of most important control points of tumor invasion and metastasis, which is responsible for the high mortality and poor prognosis [4, 5].
Although not yet completely understood, tumor angiogenesis is a complicated progression involving the participation of tumor cells, tumor microenvironment and the orchestrated regulation of multiple signaling pathways [6]. Numerous angiogenic factors have been identified including ANG2, HIF1α, TGFβ, DLL4, VEGF, PLGF and bFGF-1, among which the pro-angiogenic signaling molecule VEGF and its cognate receptors have emerged as the major regulator of angiogenesis [7]. High expression of VEGF and the increased chaotic tumor blood vessels are closely related to the invasiveness, metastasis and poor prognosis of CRC, as well as many other tumors [8, 9]. Thus, the key anti-angiogenesis therapy in CRC focus largely on inhibiting VEGF/VEGFR signaling [10]. For example, bevacizumab (Avastin), the monoclonal antibody of VEGF, is the first FDA proved anti-angiogenic drug for the treatment of metastatic CRC. Although many anti-angiogenic drugs targeting VEGF pathway can clinically improve the survival of most cancer patients, the primary or acquired resistance is commonly seen in some patients. Alternative mechanisms of angiogenesis still need to be disclosed [11].
Dickkopf 2 (DKK2) is a member of dickkopf family (DKK1, 2, 3 and 4), which is identified as a secreated modulator of Wnt via binding to the lipoprotein receptor-related protein 5/6 (LRP5/6) component of the Wnt receptor complex [12-14]. DKK2 can either stimulate or inhibit Wnt signalling, depending on its molecular context and binding partners including LRP6 and cofactors [14, 15]. DKK2 has been studied in several malignancies involving in tumor cell survival, proliferation, migration and invasion [16-18]. However, roles of DKK2 are controversial in different cancer types and its precise cellular function remains elusive. Silencing of DKK2 by methylation induces cell cycle arrest and apoptosis through suppressing Wnt/β-catenin signaling in breast cancer [19]. DKK2 is also epigenetically suppressed to reduce the invasion and induce apoptosis of tumor cells in renal carcinoma [17]. On the contrary, DKK2 stimulates tumor growth and metastasis through the transcriptional upregulation of MMP1 in Ewing's Sarcoma [16]. DKK2 also promotes proliferation and invasion via Wnt signalling pathway in prostate cancer [20]. In addition, DKK2 treatment promotes angiogenesis in human and ischemic animal models [21]. Furthermore, it has been identified that DKK2 is also involved in tumor angiogenesis [22, 23].
In the present study, we demonstrated the critical roles that DKK2 played in CRC metastasis and angiogenesis both in vitro and vivo. Importantly, we found that DKK2 could interact with LRP6 to accelerate tumor aerobic glycolysis and the following accumulation of lactate in the microenvironment, which stimulate angiogenesis through a traditional VEGF/VEGFR independent pathway. Furthermore, we found that the expression of DKK2 is directly controlled by the methylation of miR-493-5p. In summary, our study has provided a novel mechanism for colorectal metastasis and angiogenesis, suggesting that DKK2 might be a potential anti-angiogenic target in clinical treatment of the advanced CRC patients.
Materials and Methods
Cell culture and treatment
A human colon epithelial cell line NCM460 and seven CRC cell lines including LS174T, RKO, HCT116, SW480, HT29, SW620 and LOVO were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and maintained as previously described [24]. All cell lines used in this study obtained certificates within four years that authenticated by performing short tandem repeat (STR) profiling, and experiments were performed in cells propagated less than 6 months after resuscitation. RPMI 1640 (Hyclone; Logan, Utah, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco-BRL, Invitrogen; Paisley, UK) was used to culture those cells at 37 ℃ with a humidity of 5% CO2.
Glucose and Lactate were purchased from sigma (sigma-aldrich, USA), and added in the culture cells for 24 h at a concentration of 15 mM and 100 mM, separately. Recombinant human DKK2 was obtained from Peprotech (Rocky Hill, NJ, USA) and administrated to the culture medium at a final concentration of 2 mg/ml. Inhibitors including PI3K inhibitor LY294002 (Cell Signal Technology, Danvers, MA), Wnt inhibitor IWR-1 (Selleck, Shanghai, China), and MCT inhibitor 7ACC2 (MedChemExpress, Monmouth Junction, NJ) were added to cultured CRC cells for 24 h at a concentration of 10 mM, 25 μM and 10 μM separately. One week after the formation of subcutaneous tumors, 25 μM 7ACC2 was injected subcutaneously beside the tumor once a week. Moreover, Paramycin and 5-Aza were purchased from sigma (sigma-aldrich, USA) and added to the culture cells at a final concentration of 10 μM for 12 h and 5 μM/L for 24 h, separately.
DKK2 overexpressed LV-DKK2 vector and DKK2 silencing sh-DKK2 vector were constructed in our lab. Si-DKK2, miR-493-5p mimic, nonspecific miR control, anti-miR-493-5p, and a nonspecific anti-miR control were purchased from GenePharma (Shanghai, China). CRC cells were transfected with 1 mg of siRNA or 4 μg cDNA in reduced serum medium (OPTI-MEM-I; Invitrogen) at a density of 0.5×105 cells/ml according to the manufacturer's protocol.
Transwell assay
Cell Migration Assay Kit (BD Biosciences) was used to evaluate the migration of CRC. Briefly, 1×105 cells in 300 μl serum-free medium were added to the upper chamber 48 h after transient transfection. Meanwhile, 700 μl medium with 20% FBS in the lower chamber acted as a chemoattractant. Subsequently, cells were incubated in this system for 24-48 h at 37 ℃. After removing non-invading cells by cotton swabs, migrated cells were fixed with pre-cold methanol and stained with 2% Giemsa solution. Finally, stained cells were counted in at least three randomly selected fields with 100X magnification under a microscope to minimize the bias.
Western blot
RIPA lysis buffer with protease inhibitor cocktail was used to extract total proteins. Proteins were quantified by BCA protein assay kit (Pierce, KeyGEN BioTECH, Jiangsu, China) before separating by SDS-PAGE gel and transferring onto the PVDF membrane (Millipore, Darmstadt, Germany). Tris buffer containing 0.1% Tween-20 and 5% nonfat milk was used to block the membrane at 4 ℃. Rabbit antibodies to DKK2 (1:2500 Santa Cruz, California, USA), p-AKT (Ser473), AKT (1:1000; CST, Danvers, MA, USA) and mTOR (1:1000; CST, Danvers, MA) were used to incubate with the membrane overnight, which is followed by the treatment of HRP-conjugated secondary antibody (1:10000; CST, Danvers, MA, USA). The signal was detected by the enhanced chemiluminescence detection system (Tennon5200, shanghai, China) as described by the manufacturer.
Human umbilical vein endothelial cells (HUVEC) tubule formation assay
Tubule formation assay for HUVEC was performed as previously described [25]. Briefly, a 24-well plate was polymerized by Matrigel (BD Biosciences, Bedford, MA) for 30 min at 37 ℃. HUVECs (2×104) were incubated in 200μl conditioned medium (CM) for 12 h before image taking. The capillary tubes were quantified under a 100X bright-field microscope, by measuring the total lengths of the completed tubule structure. Three independent experiments were required for each treatment.
Chicken chorioallantoic membrane (CAM) assay
Day-6 fertilized chicken eggs (Yueqin Breeding Co. Ltd, Guangdong, China) were chosen to perform the CAM assay [26]. To expose the CAM, a window about 1.0 cm in diameter was opened in the eggshell. A sterile rubber ring in 0.5 cm diameter was placed on the CAM before 100 μl conditioned medium (CM) was added. The window was closed using a piece of steriled adhesive tape, and eggs were placed in a 37 ℃ incubator with 80-90% relative humidity for 2-3 days. CAMs were fixed by stationary solution (methanol: acetone=1:1) for 15 min before it was cut and harvested. Photos were taken by a digital camera (Cannon, Japan) and the effects of CM on angiogenesis were assessed through assessing the number of second- and third-order vessels.
Luciferase Assay
Lipofectamine 3000 transfection reagent (Life Technologies, Carlsbad, USA) was used to transfect luciferase reporter vectors into 293FT and SW480 cells placed in 6-well plates. Then, cells were split into 12-well plates and incubated for 24 h in duplicates. Culture supernatant was harvested for luciferase assays using a luciferase assay kit (Promega, Madison, Wisconsin, USA) and the luciferase activity was measured with PerkinElmer 2030 Multilabel Reader (PerkinElmer, Waltham, Massachusetts, USA).
Animal studies
All experiments related to animal studies were endorsed by the Animal Care and Use Committee of The Southern Medical University and all procedures followed the NIH guidelines for animal handling. Twenty-four six-weeks-old female nu/nu mice were divided into four groups and housed with free access to food and water. To verify the effects of DKK2 on colorectal cancer growth in vivo, mice were injected subcutaneously with DKK2 overexpressed SW620 and RKO cells (5×106) that dissolved in phosphate-buffered saline (PBS). One month later, those mice were sacrificed and tumors were removed. Both the volume and the weight of tumors were calculated.
Statistical analysis
Three independent experiments were performed for each treatment and all data was presented as means ± standard deviation. Student's t-test was used to analysis the difference within only two groups and one-way analysis of variance (ANOVA) are chosen when three or more groups were compared. P < 0.05 was considered statistically significant. Statistic analyses of clinical samples were carried out by SPSS19.0 software.
Results
Overexpression of DKK2 is correlated with the progression and poor prognosis of CRC
We demonstrated that DKK2 is a differentially expressed gene in metastatic CRC tissues for the first time through analyzing a high-throughput microarray dataset (NCBI/GEO/GSE113296; n=8, Figure 1A) of eight pairs of CRC tissues w/o metastasis submitted by our lab. Western blot and IHC analyses were adopted to study the expression of DKK2 in CRC tissues and their adjacent non-tumor mucosa. Twelve pairs of fresh CRC tissues with non-tumor mucosa were measured, and DKK2 protein is dramatically upregulated in CRC tissues compared with paired non-cancerous colorectal tissues (Figure 1B, P<0.01). IHC results indicated that DKK2 was significantly upregulated in 78.7% (37/47) of CRC tissues examined compared to that in 32.1% (9/28) of the adjacent non-tumor colorectal tissues (Figure 1C, P<0.01).
DKK2 overexpression is correlated with advanced progression and poor prognosis of CRC. (A) Heat map depicting the expression of DKK2 in eight pairs of human nmCRC and mCRC tissues. Yellow and purple indicate high and low DKK2 expression. (B) Expression of DKK2 protein was detected in twelve paired fresh human CRC tissues with paired noncancerous mucosa.; Scatter diagram on the right panel represents relative DKK2 expression in CRC tissues and paired normal tissues (P<0.01). (C) IHC analysis of two represented cases shows the expression of DKK2 protein in CRC tissues (T) and adjacent normal mucosa (N). Right Panel represents percentage of high and low DKK2 expression in both CRC tissues and adjacent normal mucosa (P<0.01). Scale bar represents 50 μm. (D) Expression of DKK2 protein in twenty-eight pairs of fresh human non-metastatic and metastatic CRC tissues. Visualization of four cases was shown. Right panel represents relative DKK2 expression in nmCRC and mCRC tissues (P=0.004). (E) IHC analysis of the expression of DKK2 protein in nmCRC and mCRC tissues. Right Panel represents percentage of high and low DKK2 expression in nmCRC and mCRC tissues (P=0.007). Scale bar represents 50 μm. (F) Kaplan-Meier survival curves and univariate analyses (log-rank) for CRC patients with low DKK2 expression versus high DKK2 expression through analyzing a published CRC data set (NCBI/GEO/GSE 87211, n=351).
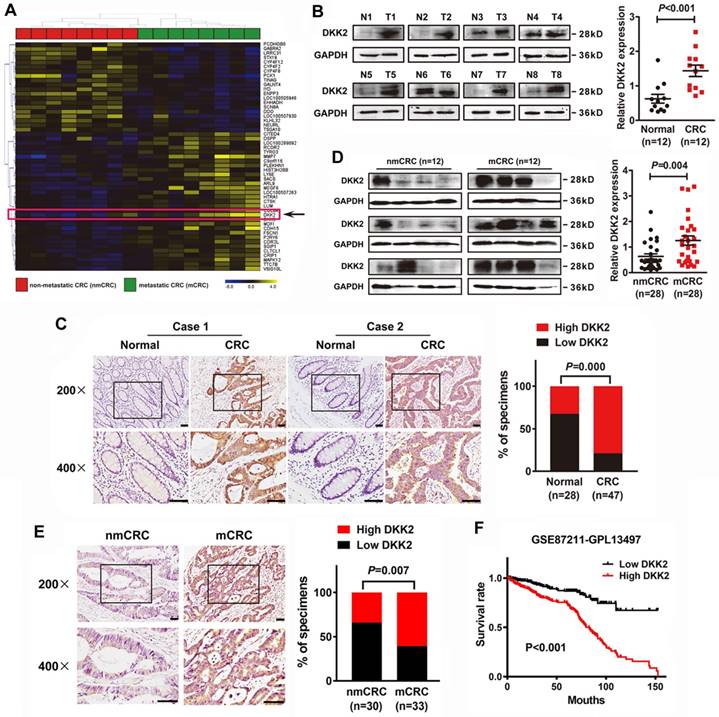
Moreover, western blot analysis of 28 pairs of fresh CRC tissues w/o metastasis further demonstrated that DKK2 protein is overexpressed in metastatic CRC (mCRC) tissues compared with non-metastatic (nmCRC) tissues (P = 0.004, Figure 1D). IHC assay was performed in 30 nmCRC tissues and 33 mCRC tissues to evaluate the clinical significance of DKK2. The IHC staining was scored semi-quantitatively based on the positive staining intensity in tumor cells. Negative (-) and weak (+) samples were scored as low DKK2, while moderate (++) and strong (+++) samples were scored as high DKK2 (Figure S1). DKK2 was highly expressed in 33.3% (10/30) of nmCRC colorectal tissues and 60.6% (20/33) of mCRC samples (P=0.007, Figure 1E). Kaplan-Meier survival analyses revealed that DKK2 expression is closely correlated with patients' survival rate and length of a published CRC data set (NCBI/GEO/GSE 87211, n=351). CRC patients with high DKK2 expression have a worse outcome (Figure 1F).
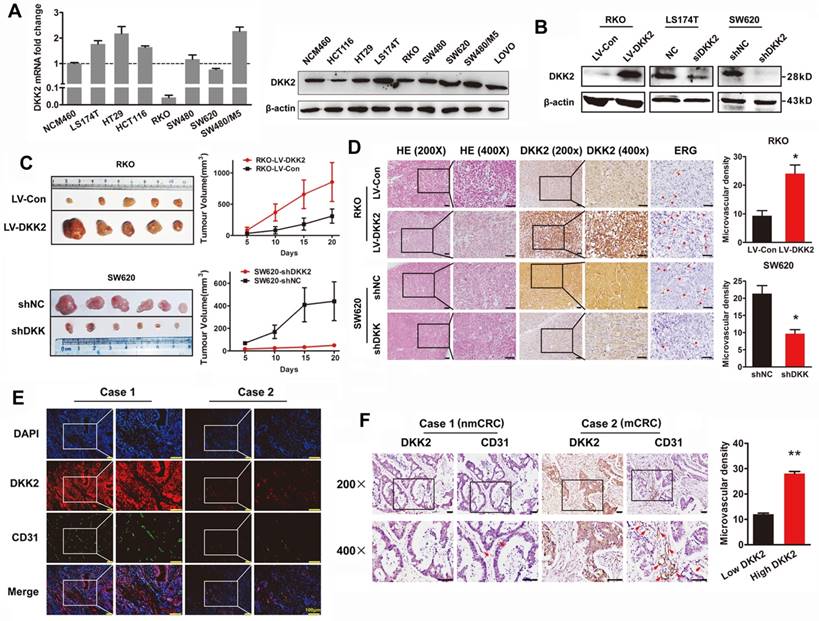
DKK2 promotes the progression of CRC cells via stimulating angiogenesis both in vitro and in vivo. (A) Expression of DKK2 mRNA and protein was detected in NCM460 and seven different colorectal cancer cell lines. Each bar represented the mean ± SD (n≥3). (B) Western blot assay was used to verify the successful construction of DKK2 overexpression and silencing CRC cells. (C) DKK2 stable overexpression RKO cells (n=5) and DKK2 stable silencing SW620 cells (n=6) were subcutaneously injected into nude mice. Both the volume and weight of subcutaneous tumor were shown in the right panel. (D) H&E and IHC staining were used to detect the expression of DKK2 and ERG in indicated subcutaneous tumors of nude mice. Bars of the right panel represent the microvascular density. The asterisk (*) indicates P < 0.05. Scale bar represents 50 μm. (E) The expression of DKK2 and CD31 in human CRC tissues we assessed by immunofluorescence staining. Visualization of two cases was shown. (F) IHC analysis demonstrated the expression of DKK2 and CD31 in human CRC tissues. Two representative cases were shown. Microvascular density was presented on the right panel. The asterisk (**) indicates P < 0.01. Scale bar represents 50 μm.
DKK2 promotes the progression of CRC cells via stimulating angiogenesis both in vitro and in vivo
The expression of DKK2 was detected in normal colon cells and seven different CRC cell lines. Real-time PCR and western blot analysis indicated that DKK2 is highly expressed in CRC cell lines including LS174T, HT29 and SW480/M5, compared with noncancerous human colon epithelial cell line NCM460 (Figure 2A). In order to investigate the biological behaviors of DKK2 in CRC progression, We successfully constructed the DKK2 overexpression lentivirus (LV-DKK2) and interference vector (shDKK2), as well as synthesized DKK2 siRNA (Figure 2B and Figure S2-3). Exogenous introduction of DKK2 dramatically increased the migration and motility ability of DKK2 low-expressed RKO cells in transwell assay and wound-healing assay. On the contrary, siDKK2 and shDKK2-mediated silencing of endogenous DKK2 inhibited the migratory ability of LS174T CRC cells (Figure S4). Although the in vitro CCK8 assay and the in vivo IHC detection of Ki-67 in subcutaneous tumor did not support that DKK2 obtained a stimulatory role in CRC cell proliferation (Figure S5), DKK2 did promote subcutaneous tumor growth. The RKO/LV-DKK2 group obtains the subcutaneous tumors with larger volume and weight, compared with that of RKO/LV-NC group (Figure 2C).
As DKK2 did not mediate CRC progression through stimulating tumor cell proliferation, other mechanisms still needed to be disclosed. Angiogenesis is proposed to play a vital role in DKK2 mediated CRC progression. IHC staining was performed in subcutaneous tumors to detect the expression of DKK2 and endothelial transcription factor (ERG). Expression of ERG is positively correlated with that of DKK2, as well as the formation of microvessels. Consistently, silencing of DKK2 is accompanied with low ERG expression and the reduced microvascular density (Figure 2D). Moreover, we detected the expression of DKK2 and CD31 proteins using both confocal immunofluorescence and immunohistochemical staining in paraffin-embedded tissues from 120 CRC patients. Results showed that both DKK2 protein and microvascular density indicated by the expression of CD31 are significantly higher in mCRC tissues compared with those in nmCRC tissues (Figure 2E-F). Results above suggested that overexpression of DKK2 might related to tumor angiogenesis.
DKK2 promotes angiogenesis through a non-classical angiogenic pathway
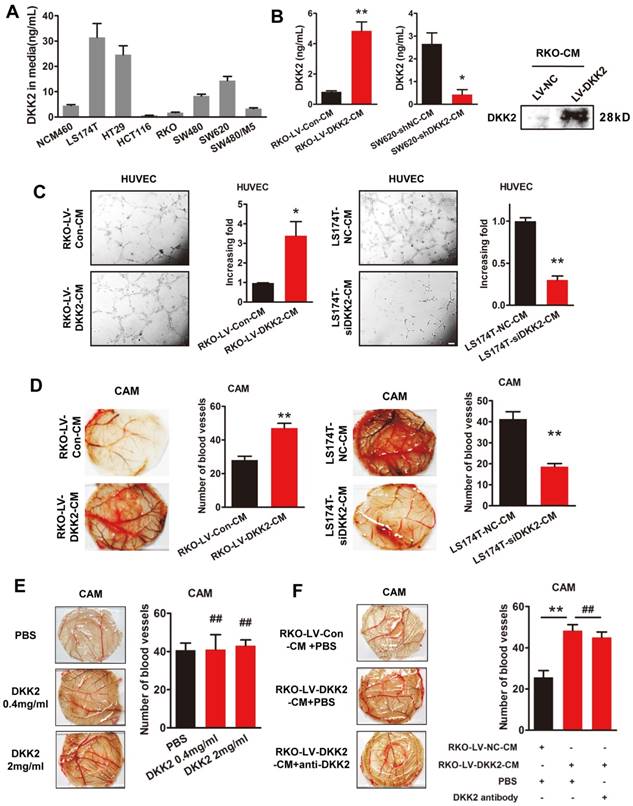
We studied the effects of DKK2 on the expression of classical angiogenic factors including ANG2, HIF1α, TGFβ, DLL4, VEGF, PLGF and bFGF-1. However, no obvious response to DKK2 was detected by real-time PCR assay (Figure S6). DKK2 might promote angiogenesis through a non-classical angiogenic pathway. As a secretory protein, we detect the secretion of DDK2 in the culture medium of different CRC cells by ELISA. High concentration of DKK2 was detected in the culture medium of LS174T, HT29 and SW620 CRC cells (Figure 3A). Overexpression of DKK2 increased the secretion of DKK2 in RKO cells while silencing of DKK2 decrease the secretion of DKK2 in SW620 cells detected by western blot assay and ELSIA (Figure 3B). As the migration of endothelial cells is critical for angiogenesis, wound-healing assay and transwell cell assay was used to assess the effects of secreted DKK2 on HUVECs migration. Both the tubule formation of HUVECs and CAM assays was adopted to further elucidate the potential function of DKK2 in angiogenesis. Culture medium of DKK2 overexpressed RKO cells significantly promoted HUVECs' migration and motility (Figure S7A & C). Meanwhile, culture medium of DKK2 silencing SW620 cells inhibited HUVECs' migration and motility (Figure S7B & D). Results revealed that DKK2 overexpressed culture medium strongly accelerated the tubule formation of HUVECs and angiogenesis in CAM assay (Figure 3C, Left panel; Figure 3D Left panel). These effects were dramatically inhibited when DKK2 was silenced (Figure 3C, Right panel; Figure 3D Right panel).
Interestingly, only the culture medium of DKK2 overexpressed RKO cells could stimulate the angiogenesis of CAM, recombinant DKK2 alone did not bring that effect (Figure 3E-F). Moreover, DKK2 antibody did not influence the stimulatory effect of DKK2 overexpressed culture medium on the angiogenesis of CAM. It is obvious that other factors, other than DKK2 in the culture medium, must exist to stimulate CRC angiogenesis.
DKK2 promotes CRC angiogenesis through stimulating lactate production in a high rate of aerobic glycolysis
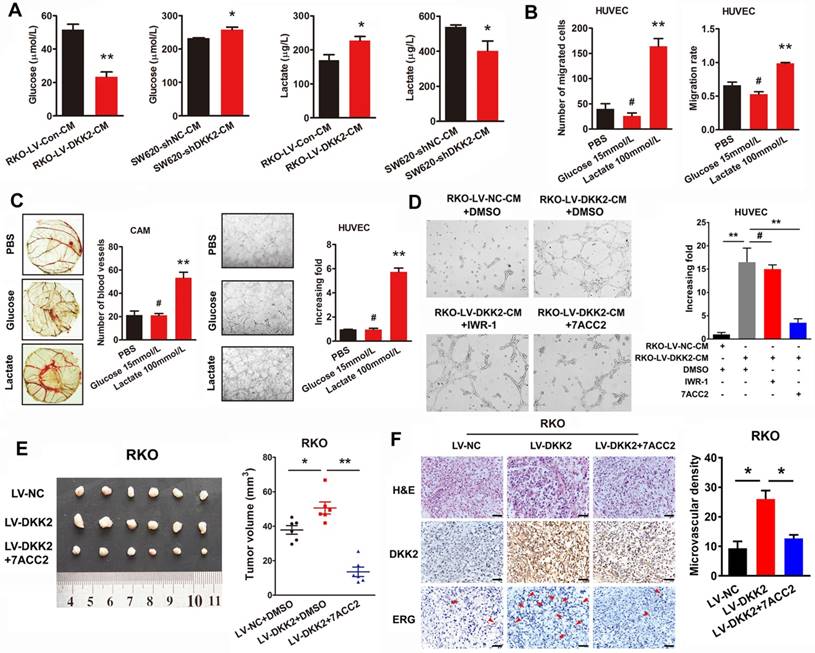
Tumor microenvironment has been identified playing a vital role on tumor progression. According to the theory of Warburg effect, different from normal cells, energies produced by tumor cells are predominantly through a high efficiency glycolysis followed by lactate accumulation even with the existence of oxygen. Interestingly, we found that lactate, as the end product of tumor glycolysis, plays an indispensable role in tumor tubule formation. The concentration of glucose and lactate in the culture medium of control, DKK2 overexpressed and DKK2 silencing CRC cells was measured by ELISA assay. As expected, a lower concentration of glucose and a higher concentration of lactate were detected in the culture medium of DKK2 overexpressed RKO cells compared with that of control RKO cells. Consistently, a higher glucose concentration and a lower lactate concentration were observed in the culture medium of DKK2 silencing SW620 cells than that in the control SW620 cells (Figure 4A). Further in vitro transwell and wound-healing assays indicated that the treatment of lactate, not glucose, resulted in the increased ability of cell migration and motility (Figure 4B). Moreover, lactate could promote chicken CAM angiogenesis and HUVECs tube formation at the concentration of 100 mM/L. However, glucose, again, had no effect on either chicken CAM angiogenesis or HUVECs tube formation (Figure 4C). Administration of 7ACC2, the inhibitor of MCT, which hinders the transportation of lactate out of the tumor cells and contributes to the increased PH in the microenvironment, dramatically block the stimulatory effects of DKK2 on HUVECs tube formation. On the contrary, IWR-1, the inhibitor of Wnt signaling could not reduce the tube formation of HUVECs (Figure 4D). Moreover, injection of 7ACC2 could also dramatically decrease the growth of subcutaneous tumors (Figure 4E). The IHC staining of DKK2 and ERG in subcutaneous tumor demonstrated that 7ACC2 significantly suppressed the DKK2 induced ERG expression and microvascular density (Figure 4F). All of the results indicated that DKK2 promote CRC angiogenesis by stimulating lactate secretion.
Secreted DKK2 promotes CRC angiogenesis indirectly. (A) The concentration of DKK2 was detected by ELISA kit in the culture medium of NCM460 and seven different CRC cell lines. (B) The concentration of DKK2 was detected in the culture medium of DKK2 overexpressed RKO cells and DKK2 silencing SW620 cells by ELISA (mean ± SD, n = 3). The asterisk (*) indicates P < 0.05. The asterisk (**) indicates P < 0.01. The secretion of DKK2 was also detected by Western blot (right panel). (C) Representative capillary tubule structures were shown for HUVECs treated with culture medium collected from the indicated RKO and LS174T. Bars on the right represent the fold change of tubule formation (D) Blood vessels formed in representative images of the CAM assay after CM treatment. Bars on the right represent the number of the blood vessels. The asterisk (*) indicates P < 0.05, (**) indicates P < 0.01. (E) Blood vessels formed in representative images of the CAM assay after DKK2 protein treatment at different concentrations (mean ± SD, n = 3). (##) indicates P > 0.01. (F) Blood vessels formed in representative images of the CAM assay after treatment of DKK2-overexpressed CM and DKK2 antibody (mean ± SD, n = 3). The asterisk (*) indicates P < 0.05, (**) indicates P < 0.01, (##) indicates P > 0.05.
DKK2 promotes angiogenesis through a non-classical angiogenic pathway. (A) The concentration of lactate and glucose were detected by ELISA kit in the culture medium of DKK2 overexpressing and silencing CRC cells. (B) The representative figures of HUVECs migration treated with 15mM/L glucose or 100mM/L lactate were measured by transwell assay and wound-healing assay. Bars represent numbers of migrated cells and the migration rate. (C) Left panel, the formation of blood vessels in CAM assay was shown in representative images after treatment of glucose and lactate. Bars in the right panel represent the number of blood vessels. (**) indicates P < 0.01, (#) indicates P > 0.05. Right panel, tubule formation of HUVECs was shown in representative images after treated with glucose and lactate. Bars in the right panel represent the increasing folds of tubule formation. (**) indicates P < 0.01, (#) indicates P > 0.05. (D) Effects of Wnt signaling inhibitor IWR-1 and MCT inhibitor 7ACC2 on DKK2 induced tube formation. (E) Effects of 7ACC2 on DKK2 induced subcutaneous tumor formation. (F) H&E and IHC staining were used to detect the effects of 7ACC2 on the DKK2 induced ERG expression and microvascular density in indicated subcutaneous tumors of nude mice. Bars of the right panel represent the microvascular density. The asterisk (*) indicates P < 0.05. Scale bar represents 50 μm.
DKK2 enhances lactate secretion via activating PI3K/Akt/mTOR pathway
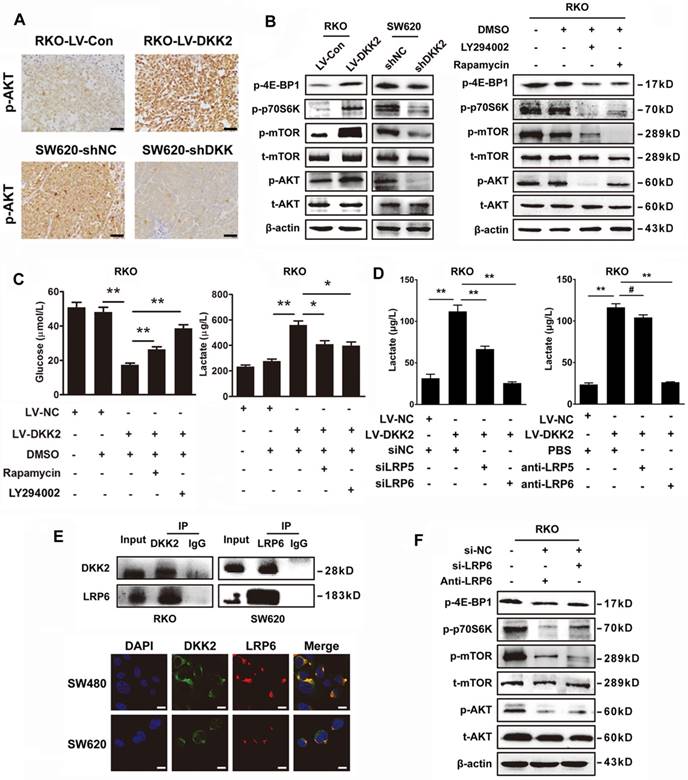
To uncover the mechanism underlying the stimulatory effects of DKK2 on lactate production and CRC angiogenesis, we detected the involvement of PI3K/Akt/mTOR signaling pathway, which had been identified vital for tumor growth and involving in the signaling of Warburg effect. We performed IHC assay to examine the expression of phosphorylated Akt (p-Akt) in subcutaneous tumors. Results showed that p-Akt level was positively correlated with that of DKK2 protein in control, DKK2 overexpressing and DKK2 silencing subcutaneous tumors (Figure 5A). Furthermore, western blot analysis was performed to detect the expression of vital signal molecules involving in PI3K/Akt/mTOR signaling pathway in DKK2 overexpressed RKO cells and DKK2 silencing SW620 cells. The changing trend of all signal molecules including p-Akt, t-Akt, p-mTOR, t-mTOR, p-4E-BP1 and p-P70S6K indicated that PI3K/Akt/mTOR signal pathway is activated in DKK2 overexpressed RKO cell and inactivated in DKK2 silencing SW620 cells (Figure 5B, left panel). Meanwhile, treatment of PI3K inhibitor LY294002 and mTOR inhibitor Rapamycin markedly prevented their downstream signaling activated by DKK2 (Figure 5B, right panel). The concentration of lactate and glucose in the culture medium were detected after treatment of LY294002 and Rapamycin. Both LY294002 and Rapamycin could dramatically reduce the secretion of lactate and the consuming of glucose. (Figure 5C). Since the PI3K/Akt/mTOR pathway could also deliver an anti-apoptotic signal in some studies, the apoptosis of control and DKK2 overexpressed RKO cells were monitored by flow cytometry w/o the appearance of LY294002. Overexpression of DKK2 could not change the apoptosis of RKO cells. Moreover, the administration of LY294002 did not attenuate the apoptosis of RKO cells induced by 5-FU (Figure S8A). It turned out that DKK2 accelerates lactate acid secretion and tumor angiogenesis via activating PI3K/Akt/mTOR signaling without altering the apoptotic status of RKO cells.
DKK2 enhances lactate secretion via activating PI3K/Akt/mTOR pathway. (A) IHC analysis of p-AKT expression in subcutaneous tumors of nude mice injected RKO-LV-DKK2 and SW620-shDKK2 cells. Scale bar represents 50 μm. (B) Left panel, the expression of PI3K/Akt/mTOR pathway members in control, DKK2 overexpressing and silencing CRC cells were detected by western blot; Right panel, Western blot analysis of PI3K/Akt/mTOR pathway members w/o the treatment of LY294002 and Rapamycin. (C). The concentration of lactate and glucose in the culture medium of control and DKK2 overexpressed RKO cells with or without the absence of LY294002 and Rapamycin. (D) The concentration of lactate and glucose in the culture medium of control and DKK2 overexpressed RKO cells with or without the absence of siLRP5/6 and anti-LRP5/6. (E) Upper panel, Endogenous interaction between DKK2 and LRP6 in CRC cells; Lower panel, the co-localization of DKK2 (green) and LRP6 (red) were assessed by immunofluorescence staining. Scale bar represents 10 μm. (F) The expression of PI3K/Akt/mTOR pathway members in RKO cells with or without LRP6 antagonist and si-LRP6.
DKK2 interacts with LPR6 to induce a Warburg effect and enhance lactate secretion
DKK2 is identified as a secreted Wnt modulator through binding to LRP5/6 component of the Wnt receptor complex. However, it is unknown whether LRP5/6 is the key receptor of DKK2 in stimulating lactate production and CRC angiogenesis. Therefore, LRP5/6 interference fragments and antibodies were used to study their effects on DKK2 induced lactate secretion. Silencing LRP6 or introduction of LRP6 antibody could dramatically reduce the secretion of lactate. However, the similar effects could not be repeated by the administration of LRP5 antibody and IWR-1, the inhibitor of Wnt signaling pathway (Figure 5D & S8B). Moreover, we performed Co-IP assay to study the protein interaction between DKK2 and LRP6 in RKO and SW620 cells. As a result, the interaction between DKK2 and the low-density LRP6 receptor was detected (Figure 5E, upper panel). This was further confirmed by the co-localization of DKK2 and LRP6 in CRC cells detected by immunofluorescence staining (Figure 5E, lower panel). In addition, western blot analysis also confirmed that silencing LRP6 or introduction of LRP6 antibody could inactivate the PI3K/AKT/mTOR signaling (Figure 5F).
Demethylation of miR-493-5p negatively regulates the expression of DKK2 in CRC cells
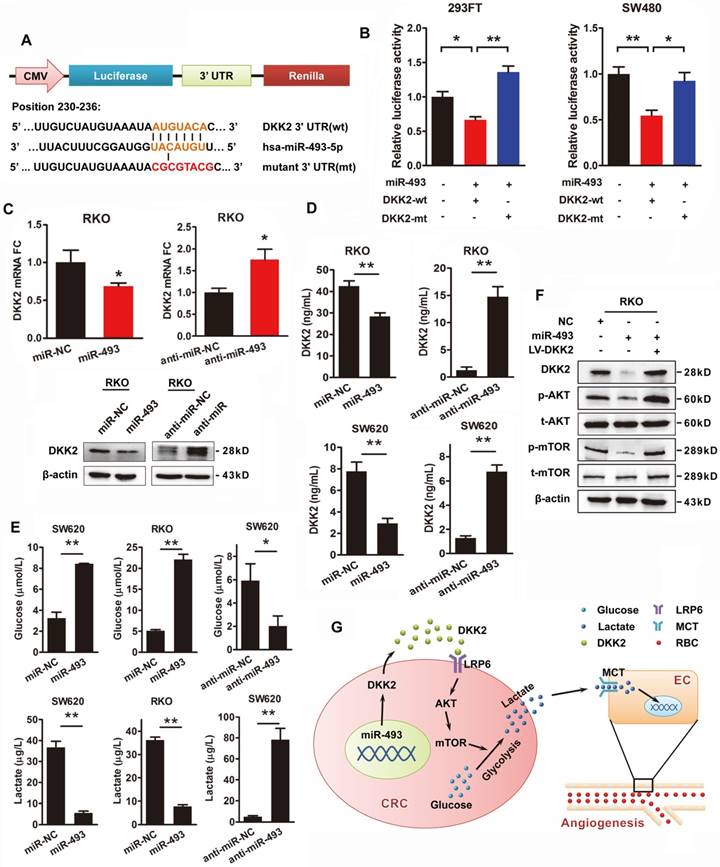
Transcriptional repression of microRNAs is thought to be a vital regulatory mechanism of gene expression. The publicly available bioinformatics algorithms TargetScan and RNAhybrid software were adopted to search the upstream miRNAs for DKK2. Both miR-128 and miR-493-5p are screened out, but only miR-493-5p was verified as an upstream regulator of DKK2 (Figure S9). DKK2 is identified as the direct target of miR-493-5p at the specific binding site of its 3′ UTR region by luciferase reporter assay. A mutation was designed in the putative-binding site (position 230-236) of the 3′UTR region (MT) (Figure 6A). Both the WT and MT fragments were cloned into luciferase report vectors and co-transfected with miR-493-5p into 293FT and SW480 cells. Overexpression of miR-493-5p decreased about 40%-50% luciferase activity of the reporter with WT 3′UTR, without influencing that with MT 3′UTR (Figure 6B). Moreover, we transfected RKO cells with miR-493-5p and its mimic oligo-nucleotides to evaluate their influence on the expression of DKK2. Anti-NC and anti-miR-493 oligonucleotides were also transfected into RKO cells to study their effects on DKK2 expression. Real-time qPCR results demonstrated that overexpression of miR-493-5p inhibited the expression of DKK2 in RKO cells. Consistently, silencing of Mir493-5p increased the expression of DKK2 in RKO cells. Western blot assay also confirmed the inhibitory role of Mir493-5p on DKK2 expression in RKO cells (Figure 6C). The in vitro transwell assay and wound-healing assay were performed to study the function of Mir493-5p on CRC progression. Overexpression of miR-493-5p resulted in a decreased cell migration and motility ability, while silencing miR-493-5p increased the migration and motility of RKO cells (Figure S10). Interestingly, the regulatory role of miR-493-5p is dependent on the methylation level of its DNA. Treatment of 5-Aza, a demethylation agent, dramatically increased the expression of miR-493-5p in both LS174T and SW620 cells (Figure S11).
MiR-493-5p inhibits secretion of DKK2 and production of lactate through PI3K/Akt/mTOR pathway
We also found that miR-493-5p negatively regulated the secretion of DKK2 in the culture medium detected by ELISA kit in RKO and SW620 cells. Meanwhile, silencing of miR-493-5p by anti-miR-493 dramatically stimulated the secretion of DKK2 (Figure 6D). The effects of miR-493-5p on the metabolism of tumor cell were further detected. Overexpression of miR-493-5p significantly increased the concentration of environmental glucose and decreased the secretion of lactate in both RKO and SW620 cells. Silencing of miR-493-5p remarkably decreased the glucose level while increased the lactate level in the culture medium of both RKO and SW620 cells (Figure 6E). Meanwhile, western blot analysis further confirmed the involvement of PI3K/AKT/mTOR pathway in the downstream signaling of miR-493-5p and DKK2 (Figure 6F).
Discussion
Activation of angiogenesis is an essential hallmark of cancer, which is required for invasive tumor growth and metastasis [4]. Angiogenic switch is turned on surprisingly early in the development of invasive cancer by the domination of proangiogenic factors [27]. Although diverse proangiogenic factors are implicated in tumor angiogenesis, VEGF is the key angiogenesis inducer. VEGF signaling via three receptor tyrosine kinases (VEGFR-1, 2, 3) on vascular endothelial cells can be regulated by multiple factors including both oncogene signalings and the hypoxic tumor environment [28]. Additionally, other proangiogenic signals, such as the fibroblast growth factor (FGF) family, is also involved in VEGF mediated tumor angiogenesis [27]. Thus, VEGF/VEGFR is considered as the primary drug target for anti-angiogenic therapeutic applications. Among these drugs are antibodies neutralizing VEGF, antibodies against VEGF receptors and tyrosine kinase inhibitors (TKIs) that blocking VEGFR-dependent signaling. Unfortunately, the benefits of anti- VEGF/VEGFR therapy do not fulfill the high expectation raised in preclinic studies [29]. Despite the striking clinical efficacy, its significant side effects and drug resistance have raised concerns of identifying novel drug targets based on unrevealed mechanisms to combat tumor angiogenesis.
DKK2 is the direct target of miR-493-5p in CRC progression. (A) The wild type and mutant DKK2 3′UTR that targeted by miR-493-5p. (B) Luciferase reporter assays were performed in 293T and SW480 cells co-transfected with wt or mt 3′UTR and miR-493-5p mimic (mean ± SD, n = 3). (C) Upper panel, the transcriptional expression of DKK2 in control, miR-493-5p, and anti-miR-493-5p transfected. Lower panel, the protein expression of DKK2 in RKO cells transfected with miR-493-5p mimic and anti-miR-493-5p. (D) The concentration of secreted DKK2 in the culture medium of RKO and SW620 cells transfected with miR-493-5p mimic or anti-miR-493-5p were detected by ELISA kit. (E) The concentration of lactate and glucose in the culture medium of RKO and SW620 cells with miR-493-5p mimic or anti- miR-493-5p were detected by ELISA kit. (F) The expression of AKT/ mTOR pathway proteins in indicated cells transfected with mir-493-5p with or without the overexpression of DKK2. (G) The sketch map of the regulation and mechanism of DKK2 mediated CRC angiogenesis.
Angiogenesis has also been demonstrated involving in the primary site tumor growth, local tissue infiltration and distant metastasis of CRC [30]. High VEGF expression and blood vessel density within CRC tissues are correlated with poor prognosis in CRC patients [8]. Bevacizumab, an anti-VEGF antibody, which is indispensable for the treatment of mCRC, is proved to be effective at first but tolerant in the later treatment. Other anti-angiogenic drugs including ramucirumab, aflibercept and regorafenib also showed similar responses in several studies on late phase CRC patients [31]. In this study, we focused on differentially expressed genes in eight pairs of nmCRC and mCRC tissues aiming to found out new tumor markers and mechanisms behind CRC metastasis. DKK2 is identified as one of the upregulated genes in metastatic CRC tissues. We demonstrated that DKK2 could promote cell migration and motility of SW620 and RKO cells in vitro. This is consistent with numerous studies that DKK2 is significantly overexpressed and contributed to tumor development in various tumor types [16, 32, 33].
Interestingly, although the CCK8 assay in vitro and the IHC detection of Ki-67 in subcutaneous tumor did not suggested a stimulatory role of DKK2 in CRC cell proliferation, DKK2 did promote subcutaneous tumor growth. Angiogenesis is proposed to play a vital role in DKK2 mediated CRC progression. As a secretory protein, DKK2 is comprised of two conserved CRDs (Cys-1 and Cys-2), and the DKK family unique N-terminal CRD (Cys-1) allows its interaction with various receptors and signaling proteins, such as LRP6 in a paracrine and autocrine manner [34]. DKK2 promoted angiogenesis in cultured human endothelial cells through activating LRP6-mediated APC/Asef2/Cdc42 to stimulate filopodial dynamics and angiogenic sprouting of endothelial cells [13, 21]. Stimulatory role of DKK2 in tumor angiogenesis has also been identified in several tumor types such as human melanoma and renal cell carcinoma [23, 35]. In the present study, we found that overexpression of DKK2 is accompanied with high microvascular density indicated by both the expression of ERG and CD31 in subcutaneous tumors. DKK2 is also positively correlated with microvascular density in 120 cases of clinical CRC tissues. Moreover, secreted DKK2 in the culture medium of CRC could stimulate the tube information of HUVEC in vitro and angiogenesis of chicken chorioallantoic membrane (CAM) in vivo. Classical angiogenic factors, at first, were considered as the targets of DKK2. However, no obvious changes was detected in the expression of those angiogenic factors including ANG2, HIF1α, TGFβ, DLL4, VEGF, PLGF and bFGF-1 in response to DKK2. Novel mechanisms of DKK2 mediated angiogenesis needs to be disclosed.
Surprisingly, only the culture medium of DKK2 overexpressed RKO cells could stimulate the angiogenesis of chicken CAM, recombinant DKK2 alone did not reach that effect. Moreover, neutralizing DKK2 could not block DKK overexpressed culture medium stimulated angiogenesis in CAM assay, which suggested an indirect effect of DKK2 on angiogenesis. Other factors driven by DKK2 in the culture medium must involve in the DKK2 promoted angiogenesis. Nowadays, increasing attention has being given to tumor metabolism and tumor microenvironment. As the Warburg effect firstly described in the 1920s, different from normal cells, aerobic glycolysis is the major energy sources of cancer cells, which is followed by the lactate accumulation [36]. Increasing evidences indicated that accumulated lactate acted as a critical regulator of cancer development, metastasis and angiogenesis [37]. Lactate is also the main cause of acidosis and immunosuppression in the microenvironment, which attribute to the tumor progression and angiogenesis [38]. In this study, we also found that lactate, the end product of tumor glycolysis, plays an indispensable role in CRC angiogenesis both in vitro and in vivo.
Several studies indicated that DKK2 is involved in the regulating of glucose metabolism. DKK2 deficiency, as well as mutations of Wnt receptor LRP5/6 is associated with dysregulation of glucose metabolism in mice [39]. Moreover, depletion of LRP5 decreases glucose uptake and lactate secretion of mammary epithelial cells [40]. It is reasonable to predict that DKK2 might promote CRC angiogenesis indirectly by lactic acid derived from DKK2 regulated tumor aerobic glycolysis. We detected the involvement of DKK2 in tumor aerobic glycolysis, which indicated by the concentration of glucose and lactic acid in the microenvironment. As expected, we verified that DKK2 protein could definitely regulate tumor glycolysis, through which glucose is converted into lactic acid and energy. Lactate has a positive feedback effect on monocarboxylate transporter (MCT), through which lactate is transported out of the tumor cells and contributing to the decreased PH in the microenvironment [41]. We also demonstrated that administration of the MCT inhibitor dramatically blockage the stimulatory effects of DKK2 on HUVECs tube formation in vitro and subcutaneous tumor growth in vivo. Furthermore, our results proved that lactate tends to be the final actor of DKK2 mediated tumor angiogenesis. Consistent with the fact that PI3K/AKT/mTOR pathway is involved in Warburg effect, AKT/mTOR pathway is also demonstrated involving in DKK2 mediated lactate production and the following CRC angiogenesis. PI3K/Akt pathway also contributes to the lactate activated sprouting of vessels from mouse aortic explants [42]. Although PI3K/Akt/mTOR pathway involves in anti-apoptotic signaling, the administration of LY294002 did not attenuate the apoptotic status of RKO cells induced by 5-FU. Moreover, overexpression of DKK2 could not change the apoptosis of RKO cells. Thus, DKK2 accelerates lactate acid secretion and tumor angiogenesis via activating PI3K/Akt/mTOR signaling without altering the apoptotic status of RKO cells.
We further verified the involvement of Wnt receptor LRP5/6 in DKK2 mediated tumor glycolysis in CRC cells. In this paper, it is LRP6, other than LRP5, that involves in DKK2 mediated lactate secretion. DKK2 might function through a Wnt/β-catenin independent pathway to influence tumor progression suggested in other studies [39]. Xiao et al. uncovered an unconventional mechanism for tumor immune evasion by DKK2 via LRP5 but independently of LRP6 and the Wnt-β-catenin pathway in melanoma [43]. IWR-1, the inhibitor of Wnt signaling could not suppress DKK2 induced tube formation, which indicating the involvement of a Wnt signaling independent pathway. Moreover, the influence of WNT/β-catenin signaling on AKT/mTOR signaling has been generally accepted [44, 45]. Unfortunately, we did not go into details of tumor metabolism to uncover the precise role that DKK2 plays on lactate production and secretion. We will focus on that in the following studies.
The upstream regulators of DKK2 in angiogenesis are also discussed in this study, but mainly focused on the regulatory role of microRNAs. We found that Mir493-5p could regulate the expression of DKK2 through directly binding to its 3' UTR. Epigenetic MiR-493-5p DNA methylation turns to be the switch of DKK2 expression and the following stimulation of tumor glycolysis. However, details still need to be verified. Clinically, high DKK2 expression is correlated with poor prognosis. As a secreted protein, it is possible to de detect the secreted DKK2 in patients blood, which might be a novel tumor marker for angiogenesis. Moreover, antibodies and small molecule inhibitors against DKK2 are of great value to the improvement of anti-angigogenic therapy.
In summary, we demonstrated the aggressive role of DKK2 in CRC metastasis and angiogenesis. Importantly, DKK2 promotes tumor angiogenesis through a novel VEGF-independent, but energy metabolism related pathway. Secreted DKK2 stimulates tumor aerobic glycolysis to increase the accumulation of lactate in the microenvironment, which is the final executor of tumor angiogenesis and metastasis. As a downstream effector of MiR-493-5p, DKK2 interacts with LRP6 receptor to accomplish its aggressive effects through activating activity of AKT/mTOR signaling pathway. The sketch map shown in Figure 6G well illustrates the mechanism of DKK2 stimulated CRC angiogenesis. Clinically, DKK2 is associated with the metastasis, angiogenesis and poor prognosis of CRC patients. Therefore, our research also proposed the diagnostic significance of DKK2, and its potential as a novel target for anti-angiogenic therapies of advanced CRC.
Supplementary Material
Supplementary figures.
Acknowledgements
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 81572813, 81773082, 81702903, 81872423), Guangdong Natural Science Foundation (2017A030310038, 2018B030311036) and Fork Ying Tung Education Foundation (161035).
Ethical Approval and Consent to participate
All experiments performed in this study are in accordance with the ethical standards of Southern Medical University and the Declaration of Helsinki. Informed consent is not applicable for all data were going to be analyzed anonymously. All animal experiments were approved by the Animal Care and Use Committee of Southern Medical University and complied with the guidelines for the ethical treatment of animals. All animal experiments involved are under the license from the Guangdong Provincial Bureau of Science.
Consent for publication
All authors consent for publication.
Authors' contributions
L. Z. led study design and prepared the manuscript; F. -L. D, R. Z and C. L carried out the experiments; H. W and W. -D. L performed statistical analysis; S. -B. Y and K. -H. Z assisted in tissue sample collection; W. -D. L and X. L performed data analysis and interpretation; M. -X. P and X. -Q. Y provided data collection. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-86
2. Moghimi-Dehkordi B, Safaee A. An overview of colorectal cancer survival rates and prognosis in Asia. World J Gastrointest Oncol. 2012;4:71-5
3. Cidon EU. The challenge of metastatic colorectal cancer. Clin Med Insights Oncol. 2010;4:55-60
4. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-74
5. Folkman J, Merler E, Abernathy C, Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med. 1971;133:275-88
6. Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol. 2010;22:617-25
7. Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011-27
8. Cao D, Hou M, Guan YS, Jiang M, Yang Y, Gou HF. Expression of HIF-1alpha and VEGF in colorectal cancer: association with clinical outcomes and prognostic implications. BMC Cancer. 2009;9:432
9. Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653-60
10. Chu QS. Aflibercept (AVE0005): an alternative strategy for inhibiting tumour angiogenesis by vascular endothelial growth factors. Expert Opin Biol Ther. 2009;9:263-71
11. Itatani Y, Kawada K, Yamamoto T, Sakai Y. Resistance to Anti-Angiogenic Therapy in Cancer-Alterations to Anti-VEGF Pathway. Int J Mol Sci. 2018:19
12. Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A. et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321-5
13. Li L, Mao J, Sun L, Liu W, Wu D. Second cysteine-rich domain of Dickkopf-2 activates canonical Wnt signaling pathway via LRP-6 independently of dishevelled. J Biol Chem. 2002;277:5977-81
14. Brott BK, Sokol SY. Regulation of Wnt/LRP signaling by distinct domains of Dickkopf proteins. Mol Cell Biol. 2002;22:6100-10
15. Mao B, Niehrs C. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene. 2003;302:179-83
16. Hauer K, Calzada-Wack J, Steiger K, Grunewald TG, Baumhoer D, Plehm S. et al. DKK2 mediates osteolysis, invasiveness, and metastatic spread in Ewing sarcoma. Cancer Res. 2013;73:967-77
17. Hirata H, Hinoda Y, Nakajima K, Kawamoto K, Kikuno N, Kawakami K. et al. Wnt antagonist gene DKK2 is epigenetically silenced and inhibits renal cancer progression through apoptotic and cell cycle pathways. Clin Cancer Res. 2009;15:5678-87
18. Zhu J, Zhang S, Gu L, Di W. Epigenetic silencing of DKK2 and Wnt signal pathway components in human ovarian carcinoma. Carcinogenesis. 2012;33:2334-43
19. Mu J, Hui T, Shao B, Li L, Du Z, Lu L. et al. Dickkopf-related protein 2 induces G0/G1 arrest and apoptosis through suppressing Wnt/beta-catenin signaling and is frequently methylated in breast cancer. Oncotarget. 2017;8:39443-59
20. Xu W, Pang K, Zhou ZG, Chen YF, Mo T, Li M. et al. Dickkopf 2 promotes proliferation and invasion via Wnt signaling in prostate cancer. Mol Med Rep. 2016;14:2283-8
21. Min JK, Park H, Choi HJ, Kim Y, Pyun BJ, Agrawal V. et al. The WNT antagonist Dickkopf2 promotes angiogenesis in rodent and human endothelial cells. J Clin Invest. 2011;121:1882-93
22. Zhao Y, Wu B, Liu Y, Xu J, Yan Q, Zhang J. Knockdown of dickkopf2 inhibits vascular endothelia growth factor expression through the Wnt/beta-catenin signaling pathway in human retinal pigment epithelial cells under hypoxic conditions. Exp Ther Med. 2018;15:4056-60
23. Park H, Jung HY, Choi HJ, Kim DY, Yoo JY, Yun CO. et al. Distinct roles of DKK1 and DKK2 in tumor angiogenesis. Angiogenesis. 2014;17:221-34
24. Zhao L, Ding YQ. [Impact of LASP-1 expression on proliferation and tumorigenesis of human colorectal cancer cell line SW480]. Zhonghua Bing Li Xue Za Zhi. 2010;39:830-4
25. Zhang F, Lu YX, Chen Q, Zou HM, Zhang JM, Hu YH. et al. Identification of NCK1 as a novel downstream effector of STAT3 in colorectal cancer metastasis and angiogenesis. Cell Signal. 2017;36:67-78
26. Celerier J, Cruz A, Lamande N, Gasc JM, Corvol P. Angiotensinogen and its cleaved derivatives inhibit angiogenesis. Hypertension. 2002;39:224-8
27. Baeriswyl V, Christofori G. The angiogenic switch in carcinogenesis. Semin Cancer Biol. 2009;19:329-37
28. Ferrara N. Vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2009;29:789-91
29. Sitohy B, Nagy JA, Dvorak HF. Anti-VEGF/VEGFR therapy for cancer: reassessing the target. Cancer Res. 2012;72:1909-14
30. Cantelmo AR, Pircher A, Kalucka J, Carmeliet P. Vessel pruning or healing: endothelial metabolism as a novel target? Expert Opin Ther Targets. 2017;21:239-47
31. Seeber A, Gunsilius E, Gastl G, Pircher A. Anti-Angiogenics: Their Value in Colorectal Cancer Therapy. Oncol Res Treat. 2018;41:188-93
32. Matsui A, Yamaguchi T, Maekawa S, Miyazaki C, Takano S, Uetake T. et al. DICKKOPF-4 and -2 genes are upregulated in human colorectal cancer. Cancer Sci. 2009;100:1923-30
33. Wang H, Duan XL, Qi XL, Meng L, Xu YS, Wu T. et al. Concurrent Hypermethylation of SFRP2 and DKK2 Activates the Wnt/beta-Catenin Pathway and Is Associated with Poor Prognosis in Patients with Gastric Cancer. Mol Cells. 2017;40:45-53
34. Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683-6
35. Choi HJ, Park H, Lee HW, Kwon YG. The Wnt pathway and the roles for its antagonists, DKKS, in angiogenesis. IUBMB Life. 2012;64:724-31
36. Warburg O, Wind F, Negelein E. The Metabolism of Tumors in the Body. J Gen Physiol. 1927;8:519-30
37. Goetze K, Walenta S, Ksiazkiewicz M, Kunz-Schughart LA, Mueller-Klieser W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int J Oncol. 2011;39:453-63
38. Justus CR, Dong L, Yang LV. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front Physiol. 2013;4:354
39. Li X, Shan J, Chang W, Kim I, Bao J, Lee HJ. et al. Chemical and genetic evidence for the involvement of Wnt antagonist Dickkopf2 in regulation of glucose metabolism. Proc Natl Acad Sci U S A. 2012;109:11402-7
40. Chin EN, Martin JA, Kim S, Fakhraldeen SA, Alexander CM. Lrp5 Has a Wnt-Independent Role in Glucose Uptake and Growth for Mammary Epithelial Cells. Mol Cell Biol. 2015;36:871-85
41. Halestrap AP. The monocarboxylate transporter family-Structure and functional characterization. IUBMB Life. 2012;64:1-9
42. Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah G, Riveros M, Perruzzi C. et al. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10:159-70
43. Xiao Q, Wu J, Wang WJ, Chen S, Zheng Y, Yu X. et al. DKK2 imparts tumor immunity evasion through beta-catenin-independent suppression of cytotoxic immune-cell activation. Nat Med. 2018;24:262-70
44. Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem. 2005;280:41342-51
45. Lee JY, Kang MB, Jang SH, Qian T, Kim HJ, Kim CH. et al. Id-1 activates Akt-mediated Wnt signaling and p27(Kip1) phosphorylation through PTEN inhibition. Oncogene. 2009;28:824-31
Author contact
![]() Corresponding author: Liang Zhao (zhaol828com), Department of Pathology, Nanfang Hospital, Southern Medical University, Guangzhou, China. Tel./fax: +86 2062789365.
Corresponding author: Liang Zhao (zhaol828com), Department of Pathology, Nanfang Hospital, Southern Medical University, Guangzhou, China. Tel./fax: +86 2062789365.
 Global reach, higher impact
Global reach, higher impact