13.3
Impact Factor
Theranostics 2019; 9(8):2129-2142. doi:10.7150/thno.31179 This issue Cite
Research Paper
Preclinical efficacy of hK2 targeted [177Lu]hu11B6 for prostate cancer theranostics
1. Division of Oncology and Pathology, Department of Clinical Sciences Lund, Lund University, Lund, Sweden
2. Department of Medical Physics and Biomedical Engineering, Sahlgrenska University Hospital, Gothenburg, Sweden
3. Molecular Pharmacology Program, Sloan Kettering Institute, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA
4. Division of Medical Radiation Physics, Department of Clinical Sciences Lund, Lund University, Lund, Sweden
5. Department of Radiology, Washington University School of Medicine, Saint Louis, MO, 63108, USA.
6. Department of Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA.
7. Department of Radiopharmacy, Karolinska University Hospital, Stockholm, Sweden
8. Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden
9. Department of Molecular and Medical Pharmacology, David Geffen School of Medicine at University of California, Los Angeles (UCLA), CA, USA
*Equal contribution
Abstract
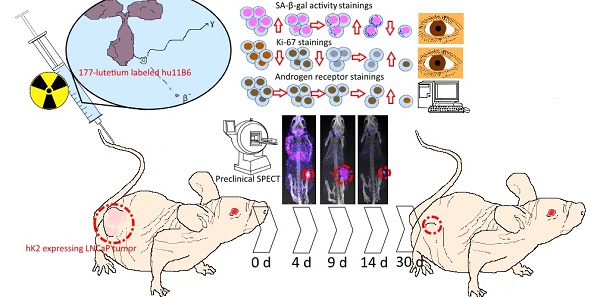
Androgen ablating drugs increase life expectancy in men with metastatic prostate cancer, but resistance inevitably develops. In a majority of these recurrent tumors, the androgen axis is reactivated in the form of increased androgen receptor (AR) expression. Targeting proteins that are expressed as a down-stream effect of AR activity is a promising rationale for management of this disease. The humanized IgG1 antibody hu11B6 internalizes into prostate and prostate cancer (PCa) cells by binding to the catalytic cleft of human kallikrein 2 (hK2), a prostate specific enzyme governed by the AR-pathway. In a previous study, hu11B6 conjugated with Actinium-225 (225Ac), a high linear energy transfer (LET) radionuclide, was shown to generate an AR-upregulation driven feed-forward mechanism that is believed to enhance therapeutic efficacy. We assessed the efficacy of hu11B6 labeled with a low LET beta-emitter, Lutetium-177 (177Lu) and investigated whether similar tumor killing and AR-enhancement is produced. Moreover, single-photon emission computed tomography (SPECT) imaging of 177Lu is quantitatively accurate and can be used to perform treatment planning. [177Lu]hu11B6 therefore has significant potential as a theranostic agent.
Materials and Methods: Subcutaneous PCa xenografts (LNCaP s.c.) were grown in male mice. Biokinetics at 4-336 h post injection and uptake as a function of the amount of hu11B6 injected at 72 h were studied. Over a 30 to 120-day treatment period the therapeutic efficacy of different activities of [177Lu]hu11B6 were assessed by volumetric tumor measurements, blood cell counts, molecular analysis of the tumor as well as SPECT/CT imaging. Organ specific mean absorbed doses were calculated, using a MIRD-scheme, based on biokinetic data and rodent specific S-factors from a modified MOBY phantom. Tumor tissues of treated xenografts were immunohistochemically (IHC) stained for Ki-67 (proliferation) and AR, SA-β-gal activity (senescence) and analyzed by digital autoradiography (DAR).
Results: Organ-to-blood and tumor-to-blood ratios were independent of hu11B6 specific activity except for the highest amount of antibody (150 µg). Tumor accumulation of [177Lu]hu11B6 peaked at 168 h with a specific uptake of 29 ± 9.1 percent injected activity per gram (%IA/g) and low accumulation in normal organs except in the submandibular gland (15 ± 4.5 %IA/g), attributed to a cross-reaction with mice kallikreins in this organ, was seen. However, SPECT imaging with therapeutic amounts of [177Lu]hu11B6 revealed no peak in tumor accumulation at 7 d, probably due to cellular retention of 177Lu and decreasing tumor volumes. For [177Lu]hu11B6 treated mice, tumor decrements of up to 4/5 of the initial tumor volume and reversible myelotoxicity with a nadir at 12 d were observed after a single injection. Tumor volume reduction correlated with injected activity and the absorbed dose. IHC revealed retained expression of AR throughout treatment and that Ki-67 staining reached a nadir at 9-14 d which coincided with high SA- β-gal activity (14 d). Quantification of nuclei staining showed that Ki-67 expression correlated negatively with activity uptake. AR expression levels in cells surviving therapy compared to previous timepoints and to controls at 30 d were significantly increased (p = 0.017).
Conclusions: This study shows that hu11B6 labeled with the low LET beta-emitting radionuclide 177Lu can deliver therapeutic absorbed doses to prostate cancer xenografts with transient hematological side-effects. The tumor response correlated with the absorbed dose both on a macro and a small scale dosimetric level. Analysis of AR staining showed that AR protein levels increased late in the study suggesting a therapeutic mechanism, a feed forward mechanism coupled to AR driven response to DNA damage or clonal lineage selection, similar to that reported in high LET alpha-particle therapy using 225Ac labeled hu11B6, however emerging at a later timepoint.
 Global reach, higher impact
Global reach, higher impact