13.3
Impact Factor
Theranostics 2019; 9(22):6485-6500. doi:10.7150/thno.34429 This issue Cite
Research Paper
Effective chemoimmunotherapy by co-delivery of doxorubicin and immune adjuvants in biodegradable nanoparticles
1. Department of Radiology, Leiden University Medical Center (LUMC);
2. Department of Immunohematology and Blood Transfusion, LUMC, Leiden, The Netherlands;
3. Department of Radiology, Erasmus MC, Rotterdam, The Netherlands.
Abstract
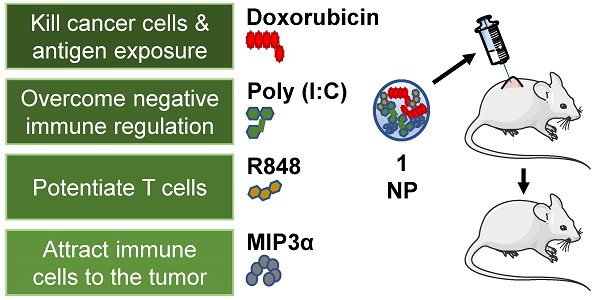
Chemoimmunotherapy is an emerging combinatorial modality for the treatment of cancers resistant to common first-line therapies, such as chemotherapy and checkpoint blockade immunotherapy. We used biodegradable nanoparticles as delivery vehicles for local, slow and sustained release of doxorubicin, two immune adjuvants and one chemokine for the treatment of resistant solid tumors.
Methods: Bio-compatible poly(lactic-co-glycolic acid)-PEG nanoparticles were synthesized in an oil/water emulsion, using a solvent evaporation-extraction method. The nanoparticles were loaded with a NIR-dye for theranostic purposes, doxorubicin cytostatic agent, poly (I:C) and R848 immune adjuvants and CCL20 chemokine. After physicochemical and in vitro characterization the nanoparticles therapeutic efficacy were carried-out on established, highly aggressive and treatment resistant TC-1 lung carcinoma and MC-38 colon adenocarcinoma models in vivo.
Results: The yielded nanoparticles average size was 180 nm and -14 mV surface charge. The combined treatment with all compounds was significantly superior than separate compounds and the compounds nanoparticle encapsulation was required for effective tumor control in vivo. The mechanistic studies confirmed strong induction of circulating cancer specific T cells upon combined treatment in blood. Analysis of the tumor microenvironment revealed a significant increase of infiltrating leukocytes upon treatment.
Conclusion: The multi-drug loaded nanoparticles mediated delivery of chemoimmunotherapy exhibited excellent therapeutic efficacy gain on two treatment resistant cancer models and is a potent candidate strategy to improve cancer therapy of solid tumors resistant to first-line therapies.
Keywords: chemoimmunotherapy, immune modulation, immune adjuvants, multi-drug nanoparticle, theragnostic.
 Global reach, higher impact
Global reach, higher impact