13.3
Impact Factor
Theranostics 2019; 9(22):6690-6705. doi:10.7150/thno.34520 This issue Cite
Research Paper
Generation of hepatic spheroids using human hepatocyte-derived liver progenitor-like cells for hepatotoxicity screening
1. Department of Interventional Oncology, Renji Hospital, Jiaotong University School of Medicine, Shanghai, China;
2. Department of Anesthesiology and Critical Care Medicine, Renji Hospital, Jiaotong University School of Medicine, Shanghai, China;
3. International Cooperation Laboratory on Signal Transduction, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai, China;
4. Celliver Biotechnology Inc., Shanghai, China;
5. Organ Transplantation Center, Changhai Hospital, Second Military Medical University, Shanghai, China.
6. Shanghai Cancer Institute, Renji Hospital, Shanghai Jiaotong University School of Medicine, 25/Ln 2200 Xietu Road, Shanghai, 200032, China.
* These authors contributed equally to this work.
Abstract
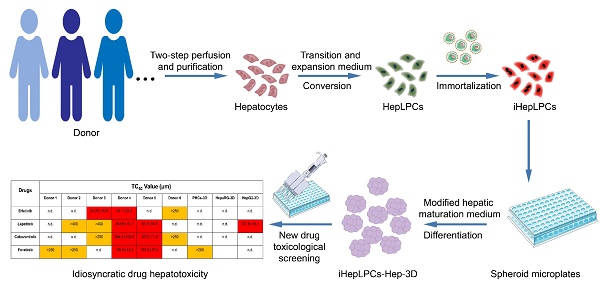
Rationale: The idiosyncratic drug-induced liver injury (iDILI) is a major cause of acute liver injury and a key challenge in late-stage drug development. Individual heterogeneity is considered to be an essential factor of iDILI. However, few in vitro model can predict heterogeneity in iDILI. We have previously shown that mouse and human hepatocytes can be converted to expandable liver progenitor-like cells in vitro (HepLPCs). However, the limited proliferation potential of human HepLPCs confines its industrial application. Here, we reported the generation of a novel hepatocyte model not only to provide unlimited cell sources for human hepatocytes but also to establish a tool for studying iDILI in vitro.
Methods: Human primary hepatocytes were isolated by modified two-step perfusion technique. The chemical reprogramming culture condition together with gene-transfer were then used to generate the immortalized HepLPC cell lines (iHepLPCs). Growth curve, doubling time, and karyotype were analyzed to evaluate the proliferation characteristics of iHepLPCs. Modified Hepatocyte Maturation Medium and 3D spheroid culture were applied to re-differentiate iHepLPCs.
Results: iHepLPCs exhibited efficient expansion for at least 40 population doublings, with a stable proliferative ability. They could easily differentiate back into metabolically functional hepatocytes in vitro within 10 days. Furthermore, under three-dimensional culture conditions, the formed hepatic spheroids showed multiple liver functions and toxicity profiles close to those of primary human hepatocytes. Importantly, we established a hepatocyte bank by generating a specific number of such cell lines. Screening for population heterogeneity allowed us to analyze the in vitro heterogeneous responses to hepatotoxicity induced by molecular targeted drugs.
Conclusions: In light of the proliferative capacity and the heterogeneity they represented, these iHepLPCs cell lines may offer assistance in studying xenobiotic metabolism as well as liver diseases in vitro.
Keywords: HepLPCs, hepatocyte spheroid, heterogeneity, idiosyncratic drug-induced liver injury, TKIs.
 Global reach, higher impact
Global reach, higher impact