13.3
Impact Factor
Theranostics 2021; 11(9):4050-4060. doi:10.7150/thno.56211 This issue Cite
Research Paper
Molecular imaging and biochemical response assessment after a single cycle of [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy in mCRPC patients who have progressed on [177Lu]Lu-PSMA-617 monotherapy
1. Department of Nuclear Medicine, Saarland University - Medical Center, Homburg, Germany
2. Department of Urology, Saarland University - Medical Center, Homburg, Germany
Abstract
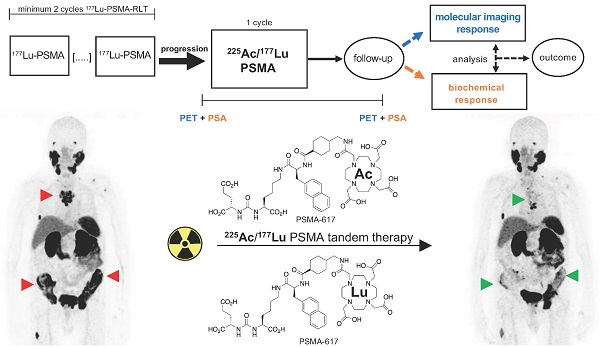
Rationale: Despite the promising results of prostate-specific membrane antigen (PSMA)-targeted 177Lu radioligand therapy in metastatic castration-resistant prostate carcinoma (mCRPC), some patients do not respond and other patients with initially good response develop resistance to this treatment. In this study, we investigated molecular imaging and biochemical responses after a single cycle of [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy in patients who had progressed on [177Lu]Lu-PSMA-617 monotherapy.
Methods: Seventeen patients with mCRPC were included in a retrospective, monocenter study. Molecular imaging-based response was assessed by modified PERCIST criteria using the whole-body total lesion PSMA (TLP) and molecular tumour volume (MTV) derived from [68Ga]Ga-PSMA-11 PET/CT. Biochemical response was evaluated according to PCWG3 criteria using the prostate-specific antigen (PSA) serum value. Concordance and correlation statistics as well as survival analyses were performed.
Results: Based on the molecular imaging-based response assessment, 5 (29.4%) patients showed partial remission and 7 (41.2%) had stable disease. The remaining 5 (29.4%) patients had further progression, four with an increase in TLP/MTV of >30% and one with stable TLP/MTV but appearance of new metastases. Based on the biochemical response assessment, 5 (29.4%), 8 (47.1%), and 4 (23.5%) patients showed partial remission, stable disease, and progressive disease, respectively. A comparison of the response assessment methods showed a concordance of 100% (17/17) between TLP and MTV and 70.6% (12/17) between TLP/MTV and PSA. Patients with partial remission, independently assessed by each method, had better overall survival (OS) than patients with either stable or progressive disease. The difference in OS was statistically significant for the molecular imaging response assessment (median OS not reached vs. 8.3 m, p = 0.044), but not for the biochemical response assessment (median OS 18.1 m vs. 9.4 m, p = 0.468).
Conclusion: Based on both assessment methods, [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy is an effective treatment for the highly challenging cohort of patients with mCRPC who have progressed on [177Lu]Lu-PSMA-617 monotherapy. Molecular imaging response and biochemical PSA response were mostly concordant, though a considerable number of cases (29.4%) were discordant. Molecular imaging response reflecting the change in total viable tumour burden appears to be superior to PSA change in estimating survival outcome after tandem therapy.
Keywords: 225Ac and 177Lu, PSMA radioligand therapy, Biochemical response, Molecular imaging response, Metastatic castration-resistant prostate cancer.
 Global reach, higher impact
Global reach, higher impact