13.3
Impact Factor
Theranostics 2021; 11(9):4122-4136. doi:10.7150/thno.53558 This issue Cite
Research Paper
MARCKS cooperates with NKAP to activate NF-kB signaling in smoke-related lung cancer
1. Department of Internal Medicine, Division of Pulmonary and Critical Care Medicine and Center for Comparative Respiratory Biology and Medicine, University of California Davis, Davis, California, USA
2. Division of Nephrology, Department of Internal Medicine, University of California Davis, Davis, California, USA
3. Comprehensive Cancer Center, University of California Davis, Davis, California, USA
4. Center for Health and the Environment and Department of Pediatrics, University of California Davis, Davis, CA, USA
Abstract
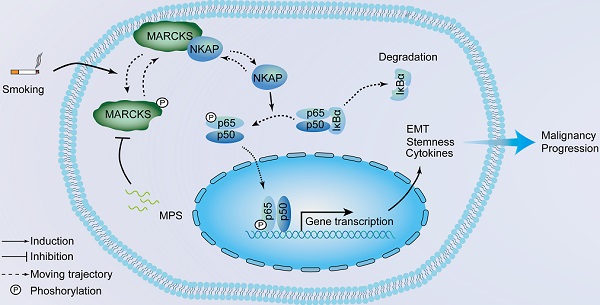
Rationale: Cigarette smoking is a major risk factor for lung cancer development and progression; however, the mechanism of how cigarette smoke activates signaling pathways in promoting cancer malignancy remains to be established. Herein, we aimed to determine the contribution of a signaling protein, myristoylated alanine-rich C kinase substrate (MARCKS), in smoke-mediated lung cancer.
Methods: We firstly examined the levels of phosphorylated MARCKS (phospho-MARCKS) in smoke-exposed human lung cancer cells and specimens as well as non-human primate airway epithelium. Next, the MARCKS-interactome and its gene networks were identified. We also used genetic and pharmacological approaches to verify the functionality and molecular mechanism of smoke-induced phospho-MARCKS.
Results: We observed that MARCKS becomes activated in airway epithelium and lung cancer cells in response to cigarette smoke. Functional proteomics revealed MARCKS protein directly binds to NF-κB-activating protein (NKAP). Following MARCKS phosphorylation at ser159 and ser163, the MARCKS-NKAP interaction was inhibited, leading to the activation of NF-κB signaling. In a screen of two cohorts of lung cancer patients, we confirmed that phospho-MARCKS is positively correlated with phospho-NF-κB (phospho-p65), and poor survival. Surprisingly, smoke-induced phospho-MARCKS upregulated the expression of pro-inflammatory cytokines, epithelial-mesenchymal transition, and stem-like properties. Conversely, targeting of MARCKS phosphorylation with MPS peptide, a specific MARCKS phosphorylation inhibitor, suppressed smoke-mediated NF-κB signaling activity, pro-inflammatory cytokines expression, aggressiveness and stemness of lung cancer cells.
Conclusion: Our results suggest that phospho-MARCKS is a novel NF-kB activator in smoke-mediated lung cancer progression and provide a promising molecular model for developing new anticancer strategies.
Keywords: MARCKS, NKAP, NF-κB, cigarette smoking, lung cancer
 Global reach, higher impact
Global reach, higher impact