13.3
Impact Factor
Theranostics 2021; 11(9):4137-4154. doi:10.7150/thno.54066 This issue Cite
Research Paper
Balancing the stability and drug activation in adaptive nanoparticles potentiates chemotherapy in multidrug-resistant cancer
1. The First Affiliated Hospital, Zhejiang University School of Medicine; NHC Key Laboratory of Combined Multi-Organ Transplantation; Key Laboratory of Organ Transplantation, Research Center for Diagnosis and Treatment of Hepatobiliary Diseases, Zhejiang Province, Hangzhou, P. R. China.
2. Institute of Pharmaceutics, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou, Zhejiang 310058, P. R. China.
3. Xingzhi College, Zhejiang Normal University, Jinhua, Zhejiang 321004, P. R. China.
Abstract
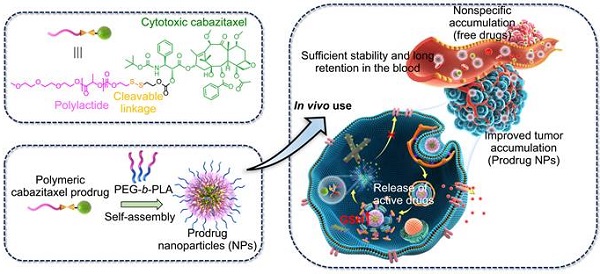
Rationale: Prodrug strategies that render the drug temporarily inactive through a cleavable linkage are able to modulate the physicochemical properties of drugs for adaptive nanoparticle (NP) formulation. Here we used cabazitaxel as a model compound to test the validity of our “balancing NP stability and specific drug activation” strategy.
Methods: Cabazitaxel is conjugated to hydrophobic polylactide fragments with varying chain lengths via a self-immolation linkage, yielding polymeric prodrugs that can be reactivated by reductive agents in cells. Following a nanoprecipitation protocol, cabazitaxel prodrugs can be stably entrapped in amphiphilic polyethylene-block-polylactide matrices to form core-shell nanotherapies with augmented colloidal stability.
Results: Upon cellular uptake followed by intracellular reduction, the NPs spontaneously release chemically unmodified cabazitaxel and exert high cytotoxicity. Studies with near-infrared dye-labeled NPs demonstrate that the nanodelivery of the prodrugs extends their systemic circulation, accompanied with increased drug concentrations at target tumor sites. In preclinical mouse xenograft models, including two paclitaxel-resistant xenograft models, the nanotherapy shows a remarkably higher efficacy in tumor suppression and an improved safety profile than free cabazitaxel.
Conclusion: Collectively, our approach enables more effective and less toxic delivery of the cabazitaxel drug, which could be a new generalizable strategy for re-engineering other toxic and water-insoluble therapeutics.
Keywords: cabazitaxel, polyprodrug, adaptive nanoformulation, drug toxicity, nanoparticle delivery
 Global reach, higher impact
Global reach, higher impact