13.3
Impact Factor
Theranostics 2021; 11(9):4155-4170. doi:10.7150/thno.54476 This issue Cite
Research Paper
Pectin supplement significantly enhanced the anti-PD-1 efficacy in tumor-bearing mice humanized with gut microbiota from patients with colorectal cancer
1. Key laboratory of whole-period monitoring and precise intervention of digestive cancer (SMHC), Minhang Hospital, Fudan University, Shanghai, 201199, P.R. China.
2. Institute of Fudan-Minhang academic health system, Minhang Hospital, Fudan University, Shanghai, 201100, P.R. China.
*Shi-Long Zhang, Yu-Qin Mao and Zheng-Yan Zhang contributed equally to this paper.
Abstract
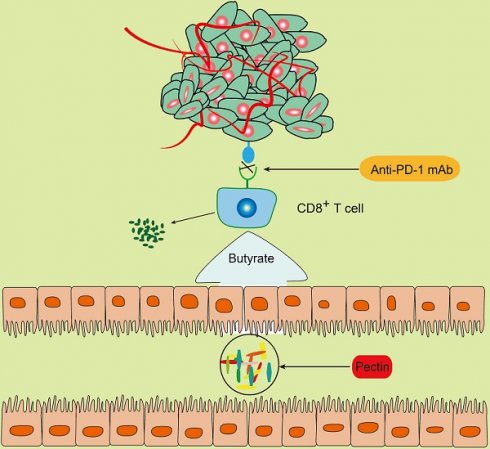
Background: Anti-PD-1-based immunotherapy has emerged as a promising therapy for several cancers. However, it only benefits a small subset of colorectal cancer (CRC) patients. Mounting data supports the pivotal role of gut microbiota in shaping immune system. Pectin, a widely consumed soluble fiber, has been reported to ameliorate the imbalance of gut microbiota. Therefore, we aimed to explore the effect and the underlying mechanisms of pectin in improving anti-PD-1 mAb efficacy.
Methods: The C57BL/6 mice were treated with a broad-spectrum antibiotic (ATB) cocktail to depleted endogenous gut microbiota and subsequently humanized with feces from healthy controls or newly diagnosed CRC patients. The antitumor efficacies of anti-PD-1 mAb combined with or without pectin were assessed using these mice. Flow cytometry and immunohistochemistry (IHC) were conducted to investigate the tumor immune microenvironment after treatment. The gut microbiota profiles and short-chain fatty acids (SCFAs) levels were determined by 16S ribosomal RNA (16S rRNA) gene sequencing and gas chromatography-mass spectrometry (GC-MS), respectively. The effect of gut microbiota on anti-PD-1 mAb efficacy after pectin supplement was further tested by fecal microbiota transplantation (FMT).
Results: The anti-PD-1 mAb efficacy was largely impaired in the mice humanized with feces from newly diagnosed CRC patients compared to those from healthy controls. However, pectin significantly enhanced the anti-PD-1 mAb efficacy in the tumor-bearing mice humanized with CRC patient gut microbiota. Flow cytometry and IHC analysis revealed increased T cell infiltration and activation in the tumor microenvironment of mice treated with anti-PD-1 mAb plus pectin. In vivo depletion of CD8+ T cells diminished the anti-tumor effect of anti-PD-1 mAb combined with pectin. 16S rRNA gene sequencing showed that pectin significantly increased gut microbial diversity and beneficially regulated microbial composition. In addition, we identified unique bacterial modules that were significantly enriched in the anti-PD-1 mAb + pectin group, which composed of butyrate-producing bacteria indicative of good response to immunotherapy. Meanwhile, GC-MS showed that pectin altered the level of SCFA butyrate. Furthermore, butyrate, a main product of dietary fiber in gut microbial fermentation, was found to be sufficient to promote T cells infiltration and thus enhance the efficacy of anti-PD-1 mAb. In addition, FMT demonstrated the effects of pectin were dependent on gut microbiota. Importantly, the beneficial effects of pectin were confirmed in the mice humanized with gut microbiota from patient with resistance to anti-PD-1 mAb.
Conclusion: Pectin facilitated the anti-PD-1 mAb efficacy in CRC via regulating the T cell infiltration in the tumor microenvironment, which was potentially mediated by the metabolite butyrate.
Keywords: colorectal cancer, gut microbiota, pectin, programmed death-1 monoclonal antibody, butyrate
 Global reach, higher impact
Global reach, higher impact