13.3
Impact Factor
Theranostics 2021; 11(14):7072-7091. doi:10.7150/thno.57803 This issue Cite
Research Paper
An innovative NRF2 nano-modulator induces lung cancer ferroptosis and elicits an immunostimulatory tumor microenvironment
1. Department of Pharmacology, National Cheng Kung University, Tainan, 70101, Taiwan.
2. Institute of Oral Medicine, National Cheng Kung University, Tainan, 70101, Taiwan.
3. Institute of Basic Medicine, National Cheng Kung University, Tainan, 70101, Taiwan.
4. Center of Applied Nanomedicine, National Cheng Kung University, Tainan, 70101, Taiwan.
5. Core Facility Center, National Cheng Kung University, Tainan 701401, Taiwan.
6. Department of Stomatology, National Cheng Kung University Hospital, Tainan, 704302, Taiwan.
*These authors contributed equally to this work.
Abstract
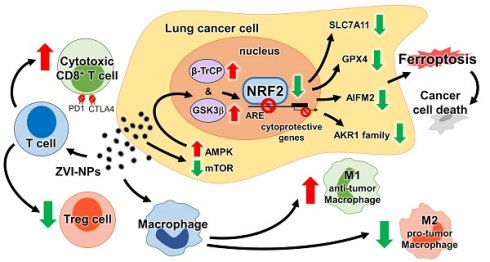
Simultaneous targeting of both the tumor microenvironment and cancer cells by a single nanomedicine has not been reported to date. Here, we report the dual properties of zero-valent-iron nanoparticle (ZVI-NP) to induce cancer-specific cytotoxicity and anti-cancer immunity.
Methods: Cancer-specific cytotoxicity induced by ZVI-NP was determined by MTT assay. Mitochondria functional assay, immunofluorescence staining, Western blot, RT-qPCR, and ChIP-qPCR assays were used to dissect the mechanism underlying ZVI-NP-induced ferroptotic cancer cell death. The therapeutic potential of ZVI-NP was evaluated in immunocompetent mice and humanized mice. Immune cell profiles of allografts and ex vivo cultured immune cells were examined by flow cytometry analysis, RT-qPCR assay, and immunofluorescence.
Results: ZVI-NP caused mitochondria dysfunction, intracellular oxidative stress, and lipid peroxidation, leading to ferroptotic death of lung cancer cells. Degradation of NRF2 by GSK3/β-TrCP through AMPK/mTOR activation was enhanced in such cancer-specific ferroptosis. In addition, ZVI-NP attenuated self-renewal ability of cancer and downregulated angiogenesis-related genes. Importantly, ZVI-NP augmented anti-tumor immunity by shifting pro-tumor M2 macrophages to anti-tumor M1, decreasing the population of regulatory T cells, downregulating PD-1 and CTLA4 in CD8+ T cells to potentiate their cytolytic activity against cancer cells, while attenuating PD-L1 expression in cancer cells in vitro and in tumor-bearing immunocompetent mice. In particular, ZVI-NPs preferentially accumulated in tumor and lung tissues, leading to prominent suppression of tumor growth and metastasis.
Conclusions: This dual-functional nanomedicine established an effective strategy to synergistically induce ferroptotic cancer cell death and reprogram the immunosuppressive microenvironment, which highlights the potential of ZVI-NP as an advanced integrated anti-cancer strategy.
Keywords: nanoparticle, NRF2, ferroptosis, tumor microenvironment, lung cancer
 Global reach, higher impact
Global reach, higher impact