13.3
Impact Factor
Theranostics 2021; 11(16):7767-7778. doi:10.7150/thno.59848 This issue Cite
Research Paper
Long-term live-cell lipid droplet-targeted biosensor development for nanoscopic tracking of lipid droplet-mitochondria contact sites
1. School of Pharmaceutical Sciences, Shandong University, Jinan 250101, PR China.
2. Institute of Materia Medica, Shandong First Medical University & Shandong Academy of Medical Sciences, Jinan 250062, PR China.
3. Shunde Hospital of Southern Medical University (The First People's Hospital of Shunde), Foshan, Guangdong 528308, PR China.
4. International Institutes of Medicine, The 4th Affiliated Hospital of Zhejiang University School of Medicine, Yiwu, Zhejiang 322000, China.
5. Shandong Academy of Pharmaceutical Science, Key Laboratory of Biopharmaceuticals, Engineering Laboratory of Polysaccharide Drugs, National-Local Joint Engineering Laboratory of Polysaccharide Drugs, Jinan 250101, PR China.
6. Advanced Medical Research Institute/Translational Medicine Core Facility of Advanced Medical Research Institute, Shandong University. Jinan 250101, PR China.
7. College of Basic Medicine, Jining Medical University, Jining 272067, PR China.
Abstract
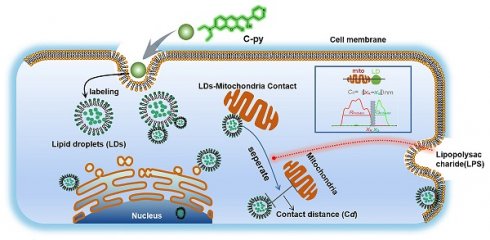
Background: Lipid droplets (LDs) establish a considerable number of contact sites with mitochondria to enable energy transfer and communication. In this study, we developed a fluorescent biosensor to image LD-mitochondria interactions at the nanoscale and further explored the function of LD-mediated matrix transmission in processes involving multi-organelle interactions.
Methods: A fluorescent probe called C-Py (C21H19N3O2, 7-(diethylamino) coumarin-3-vinyl-4-pyridine acetonitrile) was designed and synthesized. Colocalization of C-Py and the commercial LD stain Nile Red was analyzed in HeLa cells. The fluorescence stability and signal to background ratio of C-Py under structured illumination microscopy (SIM) were compared to those of the commercial probe BODIPY493/503. The cytotoxicity of C-Py was assessed using CCK-8 assays. The uptake pattern of C-Py in HeLa cells was then observed under various temperatures, metabolic levels, and endocytosis levels. Contact sites between LDs and various organelles, such as mitochondria, nuclei, and cell membrane, were imaged and quantitated using SIM. Physical changes to the contact sites between LDs and mitochondria were monitored after lipopolysaccharide induction.
Results: A LD-targeted fluorescent biosensor, C-Py, with good specificity, low background signal, excellent photostability, low cytotoxicity, and high cellular permeability was developed for tracking LD contact sites with multiple organelles using SIM. Using C-Py, the subcellular distribution and dynamic processes of LDs in living cells were observed under SIM. The formation of contact sites between LDs and multiple organelles was visualized at a resolution below ~200 nm. The number of LD-mitochondria contact sites formed was decreased by lipopolysaccharide treatment inducing an inflammatory environment.
Conclusions: C-Py provides strategies for the design of ultra-highly selective biosensors and a new tool for investigating the role and regulation of LDs in living cells at the nanoscale.
Keywords: contact sites, lipid droplets, mitochondria, extended-resolution imaging
 Global reach, higher impact
Global reach, higher impact