13.3
Impact Factor
Theranostics 2021; 11(16):7879-7895. doi:10.7150/thno.56757 This issue Cite
Research Paper
Thymosin β4 increases cardiac cell proliferation, cell engraftment, and the reparative potency of human induced-pluripotent stem cell-derived cardiomyocytes in a porcine model of acute myocardial infarction
1. National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore.
2. Department of Cardiology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China.
3. Department of Thoracic and Cardiovascular Surgery, Nanjing First Hospital, Nanjing Medical University, Nanjing, Jiangsu, China.
4. Xinxiang Medical University, Xinxiang, Henan, China.
5. Singapore Bioimaging Consortium, A*STAR, Singapore.
6. Department of Diagnostic Radiology, Singapore General Hospital, Singapore.
7. Department of Biomedical Engineering, The University of Alabama at Birmingham, Birmingham, AL, USA.
* Equal contribution first author.
Abstract
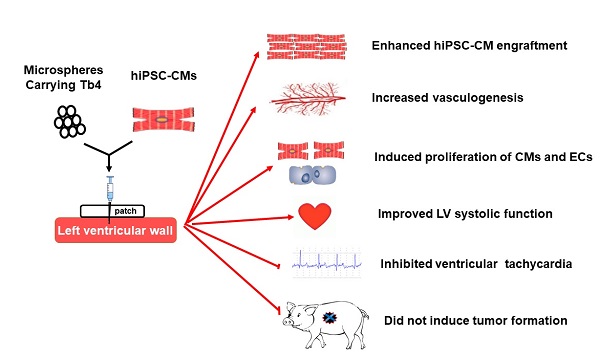
Rationale: Previous studies have shown that human embryonic stem cell-derived cardiomyocytes improved myocardial recovery when administered to infarcted pig and non-human primate hearts. However, the engraftment of intramyocardially delivered cells is poor and the effectiveness of clinically relevant doses of human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) in large animal models of myocardial injury remains unknown. Here, we determined whether thymosin β4 (Tb4) could improve the engraftment and reparative potency of transplanted hiPSC-CMs in a porcine model of myocardial infarction (MI).
Methods: Tb4 was delivered from injected gelatin microspheres, which extended the duration of Tb4 administration for up to two weeks in vitro. After MI induction, pigs were randomly distributed into 4 treatment groups: the MI Group was injected with basal medium; the Tb4 Group received gelatin microspheres carrying Tb4; the CM Group was treated with 1.2 × 108 hiPSC-CMs; and the Tb4+CM Group received both the Tb4 microspheres and hiPSC-CMs. Myocardial recovery was assessed by cardiac magnetic resonance imaging (MRI), arrhythmogenesis was monitored with implanted loop recorders, and tumorigenesis was evaluated via whole-body MRI.
Results: In vitro, 600 ng/mL of Tb4 protected cultured hiPSC-CMs from hypoxic damage by upregulating AKT activity and BcL-XL and promoted hiPSC-CM and hiPSC-EC proliferation. In infarcted pig hearts, hiPSC-CM transplantation alone had a minimal effect on myocardial recovery, but co-treatment with Tb4 significantly enhanced hiPSC-CM engraftment, induced vasculogenesis and the proliferation of cardiomyocytes and endothelial cells, improved left ventricular systolic function, and reduced infarct size. hiPSC-CM implantation did not increase incidence of ventricular arrhythmia and did not induce tumorigenesis in the immunosuppressed pigs.
Conclusions: Co-treatment with Tb4-microspheres and hiPSC-CMs was safe and enhanced the reparative potency of hiPSC-CMs for myocardial repair in a large-animal model of MI.
Keywords: Myocardial infarction, Stem cell therapy, Microsphere.
 Global reach, higher impact
Global reach, higher impact