13.3
Impact Factor
Theranostics 2022; 12(3):1061-1073. doi:10.7150/thno.65299 This issue Cite
Research Paper
Macropinocytic dextran facilitates KRAS-targeted delivery while reducing drug-induced tumor immunity depletion in pancreatic cancer
School of Pharmaceutical Sciences, Beijing Advanced Innovation Center for Structural Biology, and Key Laboratory of Bioorganic Phosphorus Chemistry & Chemical Biology (Ministry of Education), Tsinghua University, Beijing 100084, China.
* Co-first authors
Abstract
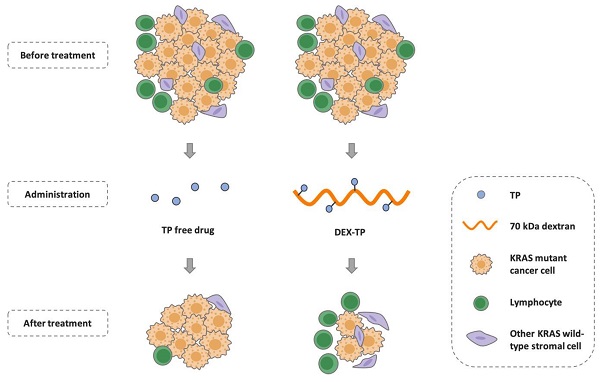
Background: Pancreatic cancer comprises not only cancer cells but also a collection of cross-talking noncancerous cells within tumor. Therefore, selective delivery of cytotoxic agents towards cancer cells and limiting the collateral damage to tumor suppressive benign cells, such as effector lymphocytes in the tumor microenvironment, is of great value.
Methods: Pancreatic cancer cells harbor oncogenic KRAS which induces a constitutively high level of macropinocytosis. Inspired by such uniquity, we sought to explore the targeting potential of dextran, a biomaterial presumed to be endocytosed in the macropinocytosis dependent manner. Cell entry preference, mechanism and subcellular sorting of dextran with different molecular weights were firstly examined. Triptolide (TP), a potent cytotoxin was then set as the model payload for dextran conjugation. KRAS selectivity and the therapeutic effects of dextran-conjugated TP were investigated via both in vitro cellular studies and in vivo tumor model assessment.
Results: Dextran, with a specific molecular weight of 70 kDa rather than other weights, was identified as a robust KRAS-responsive intracellular delivery carrier with enhanced entry upon KRAS mutation. The 70 kDa dextran-conjugated TP (DEX-TP) displayed greater efficacy and cellular deposition efficiency towards KRAS mutant cells than KRAS wild-type cells. Treatment with DEX-TP suppressed tumor progression in KRAS mutant pancreatic cancer orthotopic mouse models with reduced toxicity and significantly extended mouse survival time. Furthermore, the conjugate attained a more favorable therapeutic outcome in the tumor immune microenvironment than the free drug, preserving the fraction of T cells and their effector cytokines.
Conclusions: In summary, macropinocytic dextran was able to provide drug delivery selectivity towards KRAS mutant cancer cells and reduce tumor immunity depletion caused by the cytotoxic drug in pancreatic cancer.
Keywords: Pancreatic cancer, targeted drug delivery, KRAS mutation, dextran, tumor microenvironment
 Global reach, higher impact
Global reach, higher impact