13.3
Impact Factor
Theranostics 2022; 12(10):4536-4547. doi:10.7150/thno.71443 This issue Cite
Research Paper
Super-stable cyanine@albumin fluorophore for enhanced NIR-II bioimaging
1. State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun 130012, P.R. China
2. Joint Laboratory of Opto-Functional Theranostics in Medicine and Chemistry, The First Hospital of Jilin University, Changchun, 130021, P.R. China
3. State Key Laboratory of Precision Spectroscopy, School of Physics and Electronic Science, East China Normal University, Shanghai 200062, P.R. China
4. State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics & Center for Molecular Imaging and Translational Medicine School of Public Health, Xiamen University, Xiamen, 361102, P.R. China
5. Collaborative Innovation Center of Extreme Optics, Shanxi University, Taiyuan, Shanxi 030006, China
6. Department of Obstetrics and Gynecology, The First Hospital of Jilin University, Changchun, 130021, P.R. China.
7. Yong Loo Lin School of Medicine and Faculty of Engineering, National University of Singapore, 117597, Singapore, Singapore
Abstract
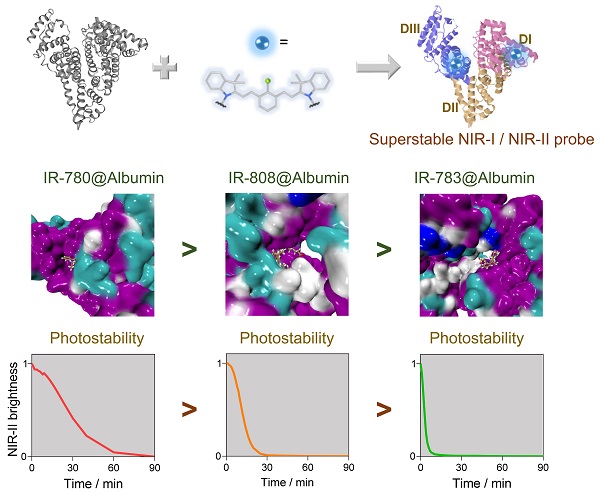
Near-infrared-II (NIR-II) dyes could be encapsulated by either exogenous or endogenous albumin to form stable complexes for deep tissue bioimaging. However, we still lack a complete understanding of the interaction mechanism of the dye@albumin complex. Studying this principle is essential to guide efficient dye synthesis and develop NIR-II probes with improved brightness, photostability, etc.
Methods: Here, we screen and test the optical and chemical properties of dye@albumin fluorophores, and systematically investigate the binding sites and the relationship between dye structures and binding degree. Super-stable cyanine dye@albumin fluorophores are rationally obtained, and we also evaluate their pharmacokinetics and long-lasting NIR-II imaging abilities.
Results: We identify several key parameters of cyanine dyes governing the supramolecular/covalent binding to albumin, including a six-membered ring with chlorine (Cl), the small size of side groups, and relatively high hydrophobicity. The tailored fluorophore (IR-780@albumin) exhibits much-improved photostability, serving as a long-lasting imaging probe for NIR-II bioimaging.
Conclusion: Our study reveals that the chloride-containing cyanine dyes with the above-screened chemical structure (e.g. IR-780) could be lodged into albumin more efficiently, producing a much more stable fluorescent probe. Our finding partly solves the photobleaching issue of clinically-available cyanine dyes, enriching the probe library for NIR-II bioimaging and imaging-guided surgery.
Keywords: NIR-II imaging, cyanine dye, albumin, super-stable NIR-II probe, covalent bond
 Global reach, higher impact
Global reach, higher impact