13.3
Impact Factor
Theranostics 2022; 12(13):5986-5994. doi:10.7150/thno.75847 This issue Cite
Research Paper
Reduced splenic uptake on 68Ga-Pentixafor-PET/CT imaging in multiple myeloma - a potential imaging biomarker for disease prognosis
1. Department of Internal Medicine II, University Hospital of Würzburg, Würzburg, Germany.
2. Department of Nuclear Medicine, University Hospital of Würzburg, Würzburg, Germany.
3. Nuclear Medicine, Faculty of Medicine, University of Augsburg, Augsburg, Germany.
4. Department of Hematology, Oncology and Cancer Immunology, Campus Benjamin Franklin, Charité - Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
5. Department of Internal Medicine V, Heidelberg University Hospital, Heidelberg, Germany.
6. Department of Nuclear Medicine, Klinikum rechts der Isar, Technische Universität München, Munich, Germany.
7. The Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
8. Technical University Munich, School of Medicine, Klinikum rechts der Isar, Clinic and Policlinic for Internal Medicine III, Munich, Germany.
9. Pharmaceutical Radiochemistry, Technical University of Munich, Munich, Germany.
10. Institute of Pathology, University of Würzburg, Würzburg, Germany.
#These authors equally contributed to this manuscript.
Received 2022-6-6; Accepted 2022-7-24; Published 2022-8-8
Abstract
Beyond being a key factor for tumor growth and metastasis in human cancer, C-X-C motif chemokine receptor 4 (CXCR4) is also highly expressed by a number of immune cells, allowing for non-invasive read-out of inflammatory activity. With two recent studies reporting on prognostic implications of the spleen signal in diffusion-weighted magnetic resonance imaging in patients with plasma cell dyscrasias, the aim of this study was to correlate splenic 68Ga-Pentixafor uptake in multiple myeloma (MM) with clinical parameters and to evaluate its prognostic impact.
Methods: Eighty-seven MM patients underwent molecular imaging with 68Ga-Pentixafor-PET/CT. Splenic CXCR4 expression was semi-quantitatively assessed by peak standardized uptake values (SUVpeak) and corresponding spleen-to-bloodpool ratios (TBR) and correlated with clinical and prognostic features as well as survival parameters.
Results: 68Ga-Pentixafor-PET/CT was visually positive in all MM patients with markedly heterogeneous tracer uptake in the spleen. CXCR4 expression determined by 68Ga-Pentixafor-PET/CT corresponded with advanced disease and was inversely associated with the number of previous treatment lines as compared to controls or untreated smouldering multiple myeloma patients (SUVpeakSpleen 4.06 ± 1.43 vs. 6.02 ± 1.16 vs. 7.33 ± 1.40; P < 0.001). Moreover, reduced splenic 68Ga-Pentixafor uptake was linked to unfavorable clinical outcome. Patients with a low SUVpeakSpleen (<3.35) experienced a significantly shorter overall survival of 5 months as compared to 62 months in patients with a high SUVpeakSpleen >5.79 (P < 0.001). Multivariate Cox analysis confirmed SUVpeakSpleen as an independent predictor of survival (HR 0.75; P = 0.009).
Conclusion: These data suggest that splenic 68Ga-Pentixafor uptake might provide prognostic information in pre-treated MM patients similar to what was reported for diffusion-weighted magnetic resonance imaging. Further research to elucidate the underlying biologic implications is warranted.
Keywords: multiple myeloma, 68Ga-Pentixafor-PET/CT, CXCR4, molecular imaging, spleen
Introduction
Multiple myeloma (MM) is a biologically heterogeneous disease characterized by the uncontrolled clonal proliferation of plasma cells predominantly within the bone marrow (BM) [1]. Both spatial and temporal heterogeneity and extramedullary involvement are recognized as additional factors to the complexity of this disease [2-4]. Recent advances in the diagnosis and treatment of MM have highlighted the importance of imaging methods not only in disease localization and staging, but also in prognostic stratification and response assessment [5]. The value of positron emission tomography/computed tomography (PET/CT) with 18F-labelled fluorodeoxyglucose (18F-FDG) has been widely investigated in varying clinical conditions and is recognized as a useful tool in the management of patients with MM [6-11]. However, 18F-FDG PET/CT also has some limitations, such as lower sensitivity in detecting bone marrow involvement in MM patients, so alternative tracers have been investigated [12-17].
C-X-C motif chemokine receptor 4 (CXCR4) is a rising ligand in molecular imaging. Beyond its physiologic role in homing of hematopoietic stem (HSCs) and immune cells [18, 19], receptor overexpression could be demonstrated in more than 30 different types of cancer including multiple myeloma, diffuse large B-cell lymphoma, breast cancer and small cell lung cancer [20-26] and could be identified as an adverse prognostic factor [20]. In MM, the interplay between receptor and its ligand stromal derived factor 1 (SDF-1α/CXCL12) serves as a stimulus of plasma cell proliferation [27].
68Ga-Pentixafor, a synthetic ligand of CXCR4, is a promising tool for in vivo positron emission tomography (PET) imaging of many solid and hematologic malignancies [25, 28-32]. Noteworthy, a wide heterogeneity not only of tumor, but also spleen CXCR4 expression could be observed [31, 33].
In this context, Rasche et al. demonstrated the spleen signal in diffusion-weighted magnetic resonance imaging (DW-MRI) to be associated with tumor burden and prognosis in 295 newly diagnosed MM (NDMM) patients [34]. Recently, this observation was confirmed in a Japanese study with 96 patients, reporting that loss of spleen visualization on DW-MRI imaging correlates with high tumor volume and poor prognosis [35]. Interestingly, heterogeneous splenic 68Ga-Pentixafor uptake could also be observed in MM patients. Therefore, the aim of this study was to evaluate the prognostic impact of 68Ga-Pentixafor uptake in the spleen of MM patients and to correlate it with clinical data and clinical outcome.
Materials and methods
Study approval was obtained from the local ethics committee of the University of Würzburg, Germany (213/13), and all patients signed written informed consent prior to 68Ga-Pentixafor-PET/CT in accordance with the Declaration of Helsinki.
Subjects and study design
Between July 2013 and May 2018, 87 patients with pre-treated MM (median of previous treatment lines 4; range, 1-12) underwent molecular imaging with 68Ga-Pentixafor-PET/CT for diagnostic work-up at the University Center of Würzburg. MM was diagnosed according to the current IMWG guidelines [36]. Detailed patients' characteristics are displayed in Table 1 and Table S2. At the time point of 68Ga-Pentixafor-PET/CT scanning, patients' treatment history, M-gradient (available in n = 75/87), serum free immunoglobulin light chains (FLC; n = 75/87) and hematological parameters (all patients) were recorded. The following hematological parameters were measured (all patients): leukocyte count, lymphocyte count, neutrophil count, monocyte count, reticulocyte count, red blood cell count (RBC), platelet count (Plt), hemoglobin (Hb), and hematocrit (Hct). Serum chemistry including lactate dehydrogenase (LDH, all patients), C-reactive protein (n = 82/87), albumin (n = 85/87), creatinine (all patients) and β2-microglobulin (n = 75/87) levels were obtained. The mean time interval time between determination of laboratory values and 68Ga-Pentixafor-PET/CT was 7±12 days. International Staging System (ISS) stages for the day of PET imaging were calculated. Additionally, interphase molecular cytogenetics based on fluorescence in situ hybridization (FISH) were available in 67/87 patients. High-risk cytogenetic abnormalities were defined by the presence of del17p, t(4;14) and t(14;16). Recent bone marrow biopsy results for assessment of malignant clonal plasma cells were available in n = 28/87 subjects. The median time between bone marrow biopsy (available in 28/87 patients) to 68Ga-Pentixafor PET/CT scan was 11±17 days (range, 0-72 days).
Nine patients with smoldering multiple myeloma (SMM; 7 males, 2 females; mean age 64±10 years) served as treatment-naive oncologic controls. In addition, five non-oncologic patients (3 males, 2 females; mean age 59±8 years) who underwent 68Ga-Pentixafor-PET/CT due to suspicion of benign Conn's adenoma were included as a “healthy” comparison group. As previously described, these patients represent the most suitable control group because the exposure of healthy volunteers to unnecessary radiation cannot be justified [37].
We used the 68Ga-Pentixafor uptake in the spleen of all MM patients and divided the patients into 3 uptake groups (<25%, 25%-75% and >75% quantiles) to determine the threshold for increased and decreased splenic uptake, respectively. SUVpeakSpleen of 3.35 and 5.79 were used to distinguish high (>5.79), intermediate (3.35-5.79) and low (<3.35) 68Ga-Pentixafor uptake in the spleen.
Patient follow-up
In order to investigate the prognostic value of splenic 68Ga-Pentixafor uptake, individual patients´ outcomes were assessed in terms of overall survival (OS) using the following formula: OS = [(Date of death)-(Date of PET imaging)].
PET/CT imaging
68Ga-Pentixafor was synthesized in-house using a fully GMP compliant automated synthesizer (GRP, Scintomics, Fürstenfeldbruck, Germany), as previously described [38]. After injection of 68Ga-Pentixafor (median 127 MBq; range 43-207 MBq) all PET/CT scans were performed on a dedicated PET/CT scanner (Siemens Biograph mCT 64; Siemens Medical Solutions, Erlangen, Germany) using standard acquisition and reconstruction protocols. For anatomical correlation and attenuation correction we subsequently acquired low-dose CT scans (35mAs, 120keV, a 512 x 512 matrix, 5 mm slice thickness, increment of 30mm/s, rotation time of 0.5s, and pitch index of 0.8). PET images were reconstructed using standard parameters (HD-PET, 3 iterations, 24 subsets, Gaussian filtering: 2 mm, resolution: axial resolution: 5 mm, in-plane resolution: 4 x 4 mm²) and corrected for attenuation, dead-time, random events and scatter.
Image analysis
PET/CT scans were visually assessed by two board-certified nuclear medicine physicians (CL and MK). First, a visual inspection of scans for elevated splenic tracer uptake was performed. For semi-quantitative analysis, standardized uptake values (SUV) for splenic uptake were determined by placing a volume of interest (VOI) with a diameter of 4 cm. The average SUV within a 10 mm circular region centered on the pixel with the highest tracer uptake was defined as SUVpeak.
For derivation of background activity, a 15 mm ROI was placed in the center of the right atrium and mean standardized uptake values (SUVbloodpool) were recorded. Afterwards, a target-to-background ratio (TBR) was calculated by dividing SUVpeakSpleen by SUVbloodpool. In addition, mean tracer uptake in a sphere with a diameter of 4 cm within the liver (SUVmeanLiver) was recorded. The radiotracer concentration in the ROIs was normalized to the injected dose per kilogram of patient's body weight to derive the SUVs.
Statistics
Statistical analyses were performed using R (version 3.6.1, R Core Team, 2019) with packages caret (version 6.0.84), rms (version 5.1-3.1), and survival (version 2.38). All results are displayed as mean ± SD or as median + range wherever appropriate. The Kaplan-Meier method was used to analyze the survival outcome of the patients. A two-tailed log-rank test was used to compare the survival outcomes between the subgroups. For bivariate correlation analyses, Spearman or Pearson correlation coefficients were calculated. A P-value of <0.05 was considered statistically significant.
Results
Splenic 68Ga-Pentixafor uptake levels show a great variability in MM patients
CXCR4-directed PET/CT imaging with 68Ga-pentixafor was visually positive in all studied MM patients (n = 87), with very heterogeneous uptake in the spleen. For assessing physiologic splenic 68Ga-Pentixafor uptake, five patients who underwent CXCR4-directed PET/CT due to suspicion of benign Conn's adenoma were included as a control group. MM patients with a SUVpeakSpleen lower than 3.35 (TBR 2.11) and above 5.79 (TBR 3.51) were considered as low-expression group and high-expression group, respectively. All other subjects were classified into an intermediate group.
SUVpeakSpleen ranged from 1.4 to 10.0 with a median of 4.5 and a median TBR of 2.97 (range, 1.3-6.2), respectively. SUVpeakSpleen and TBRs are summarized in Table S1. According to the three defined uptake categories (low, intermediate, high), 22/87 subjects qualified for the low, 42/87 for the intermediate and 23/87 for the high expression group. Detailed patients' characteristics are displayed in Table 1.
Reduced splenic 68Ga-Pentixafor uptake is associated with advanced disease stage and bone marrow insufficiency
Differences in spleen CXCR4 expression as visualized by 68Ga-Pentixafor-PET/CT could potentially be associated with tumor burden and hematopoietic reserve, as this was reported in previous studies investigating the spleen signal using MRI [34, 35]. In detail, we investigated whether splenic uptake was associated with ISS, presence of extramedullary disease (EMD), or the number of focal lesions. We found 68Ga-Pentixafor uptake of the spleen to be negatively associated with ISS stage at the time point of PET imaging (r = -0.409, P < 0.001), and percentage of malignant plasma cells in the bone marrow (r = -0.534, P = 0.003). Furthermore, presence of EMD was negatively correlated with CXCR4 expression (r = -0.352, P = 0.001). Regarding markers of acute inflammation, both serum ferritin (r = -0.462, P = 0.001) as well as C-reactive protein levels (r = -0.287, P = 0.009) were inversely correlated with the splenic PET signal (Table 2).
Patients' characteristics
| Clinical characteristics | No. of Patients (%), Total (n = 87) |
|---|---|
| Age | |
| > 65 years | 13 (14.9%) |
| ≤ 65 years | 74 (85.1%) |
| Sex | |
| Male | 60 (69.0%) |
| Female | 27 (31.0%) |
| Histologic subtype | |
| IgG | 41 (47.1%) |
| IgA | 20 (23.0%) |
| IgD | 0 (0%) |
| Light chain only | |
| Lamda | 13 (14.9%) |
| Kappa | 12 (13.8%) |
| Non-secretory | 1 (1.1%) |
| Cytogenetic high risk abnormalities on FISH | |
| del(17p) | 12 (13.8%) |
| t(4;14) | 10 (11.5%) |
| t(14;16) | 1 (1.1%) |
| Cytogenetics not available | 20 (23.0%) |
| ISS (at PET/CT imaging) | |
| I | 26 (30.0%) |
| II | 39 (44.8%) |
| III | 10 (11.5%) |
| ISS not available | 12 (13.8%) |
| R-ISS (ISS (at PET/CT imaging) | |
| I | 10 (11.5%) |
| II | 33 (37.9%) |
| III | 24 (27.6%) |
| R-ISS not available | 20 (23.0%) |
| Extramedullary disease | 23 (26.4%) |
| Prior high-dose therapy with autologous SCT | 84 (97.0%) |
| Bone marrow plasma cell percentage | 38.0 (1-90%) |
| Median (range), % | Histology available in 28/97 patients |
| Laboratory finding | |
| Anemia (<13.5g/dl) | 74 (85.0%); median 11.1 (range 7.0-15.2) |
| Elevated LDH (>250 U/I) | 34 (39.1%); median 281 (range 113-1189) |
| Hypercalcemia (>2.6 mmol/l) | 2 (2.3%); median 2.3 (range 1.5-3.2) |
| Elevated β2-microglobulin (>2.4 mg(L) | 40 (46.0%); median 4.5 (range 1.1-69) |
| β2-microglobulin not available n = 12/87 | |
| Elevated CRP (>1 mg/dl) | 26 (30.0%); median 1.4 (range 0.0-22.0) |
| Elevated creatinine (>1.09 mg/dl) | 37 (43.0%); median 1.2 (range 0.6-8.7) |
SCT: stem cell transplantation; ISS: international staging system; FISH: fluorescence staging system; CRP: C-reactive protein; LDH: lactate dehydrogenase.
In contrast, splenic 68Ga-pentixafor uptake was not correlated with myeloma CXCR4 positivity of the scan (r = -0.154, P = 0.154), high-risk cytogenetics (r = -0.127, P = 0.306), the serum level of the involved free light chain (r = -0.115, P = 0.325), the pattern of myeloma bone marrow burden (focal vs. diffuse vs. focal-on-diffuse; r = -0.017, P = 0.873) or the number of PET-positive focal lesions (r = -0.153, P = 0.156). In addition, we correlated 68Ga-pentixafor uptake in MM lesions and splenic 68Ga-pentixafor uptake and could not find a significant association (rs=-0.15; P = 0.161).
We hypothesized that differences in spleen CXCR4 expression as measured by 68Ga-Pentixafor-PET/CT could also be associated with hematopoietic insufficiency. Hence, we evaluated hematological parameters of all patients on the day of imaging and correlated SUVpeakSpleen with peripheral leukocyte count (/nl), hemoglobin levels (g/dl) and peripheral platelet count (/µl). Serum chemistry including lactate dehydrogenase (LDH), albumin, creatinine, and β2-microglobulin was also assessed. When investigating hematological parameters, we could observe a significant positive correlation of spleen 68Ga-Pentixafor uptake with higher counts for hemoglobin (r = 0.388, P < 0.001), peripheral thrombocytes (r = 0.524, P < 0.001), and peripheral leukocytes (r = 0.669, P < 0.001). Together, our observations suggest that a reduced splenic signal is mainly associated with parameters reflecting tumor burden or hematopoietic insufficiency rather than with specific tumor features. Individual values for the various laboratory parameters are displayed in Table 2.
Correlation with SUVpeakSpleen
| Variable | n | Pearson | Spearman | |||
|---|---|---|---|---|---|---|
| r | P | CI | r | P | ||
| Age | 87 | - 0.120 | 0.270 | -0,322, 0.093 | -0.101 | 0.352 |
| Number of lesions | 87 | -0.153 | 0.156 | -0.353, 0.059 | -0.152 | 0.161 |
| Extramedullary disease | 87 | -0,337 | 0.001 | -0.511, -0.136 | -0.352 | 0.001 |
| Number of therapy lines before PET/CT | 86 | -0.388 | <0.001 | -0.555, -0.192 | -0.437 | <0.001 |
| Histology (% plasmocytes in bone marrow) | 28 | -0.534 | 0.003 | -0.757, -0.202 | -0.431 | 0.022 |
| ISS at point of PET/CT | 75 | -0.409 | <0.001 | -0.576, -0.209 | -0.439 | <0.001 |
| Leucocytes | 87 | 0.315 | 0.003 | 0.112, 0.493 | 0.388 | <0.001 |
| Hemoglobin | 87 | 0.522 | <0.001 | 0.350, 0.660 | 0.524 | <0.001 |
| Thrombocytes | 87 | 0.547 | <0.001 | 0.380, 0.679 | 0.669 | <0.001 |
| Calcium | 85 | 0.131 | 0.234 | -0.085, 0.334 | 0.185 | 0.090 |
| Creatinine | 87 | -0.020 | 0.857 | -0.229, 0.192 | 0.039 | 0.717 |
| LDH | 87 | -0.199 | 0.065 | -0.393, 0.012 | -0.143 | 0.185 |
| Albumin | 85 | 0.450 | <0.001 | 0.262, 0.605 | 0.449 | <0.001 |
| Ferritin | 50 | -0.462 | 0.001 | -0.656, -0.210 | -0.623 | <0.001 |
| CRP | 82 | -0.287 | 0.009 | -0.475, -0.075 | -0.369 | 0.001 |
| ß2-microglobulin | 75 | -0.197 | 0.091 | -0.405, 0.032 | -0.407 | <0.001 |
| High risk cytogenetics | 67 | -0.127 | 0.306 | -0.356, 0.117 | -0,128 | 0.303 |
ISS international staging system; CRP C-reactive protein; LDH lactate dehydrogenase.
Dynamics of splenic 68Ga-Pentixafor uptake levels during the course of MM disease
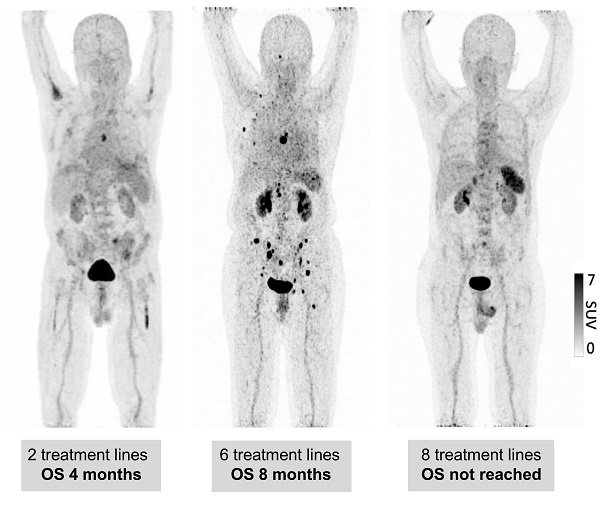
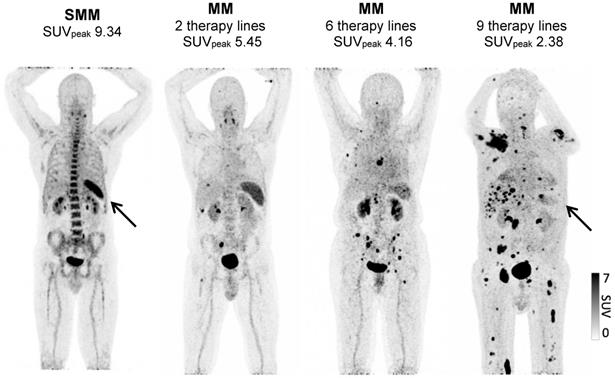
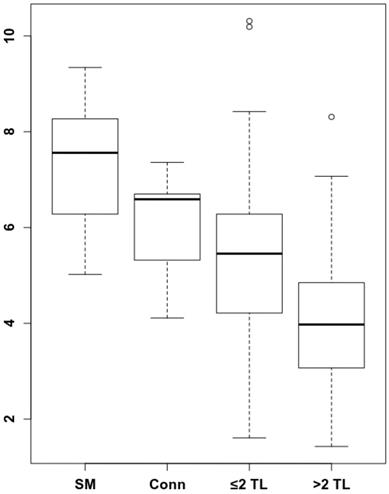
To understand by which parameters the spleen signal is altered and how this correlates with remaining survival time, we analyzed whether there are longitudinal dynamics in splenic 68Ga-Pentixafor uptake and whether there is a correlation with extramedullary hematopoiesis or tumor burden. The intensity of the splenic 68Ga-Pentixafor uptake shows dynamic changes during the course of MM disease. Patients with SMM show higher 68Ga-Pentixafor uptake in the spleen with an SUVpeakSpleen of 7.33 ± 1.40 compared to controls with an SUVpeakSpleen of 6.02 ± 1.16. Further, we hypothesized that systemic treatment could potentially influence CXCR4 receptor expression. Hence, we compared SMM patients who had not undergone treatment before imaging to prior systemically treated MM patients. We detected that spleen 68Ga-Pentixafor uptake decreases with treatment duration and an increasing number of treatment lines (Figure 1). In patients receiving one to two lines of systemic treatment, SUVpeakSpleen was 5.48 ± 1.93, and in patients receiving more than two lines of treatment, this value decreased to 4.06 ± 1.43 (Figure 2, P < 0.001).
Spleen 68Ga-Pentixafor uptake is associated with clinical outcome in MM patients
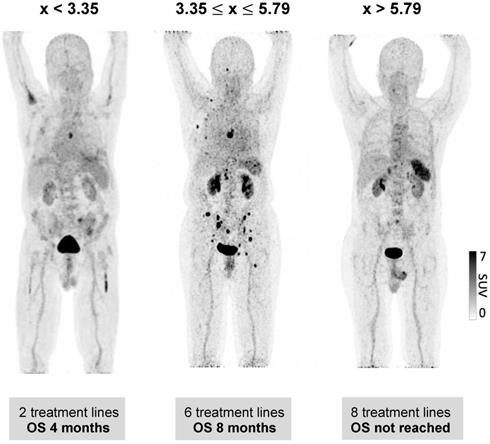
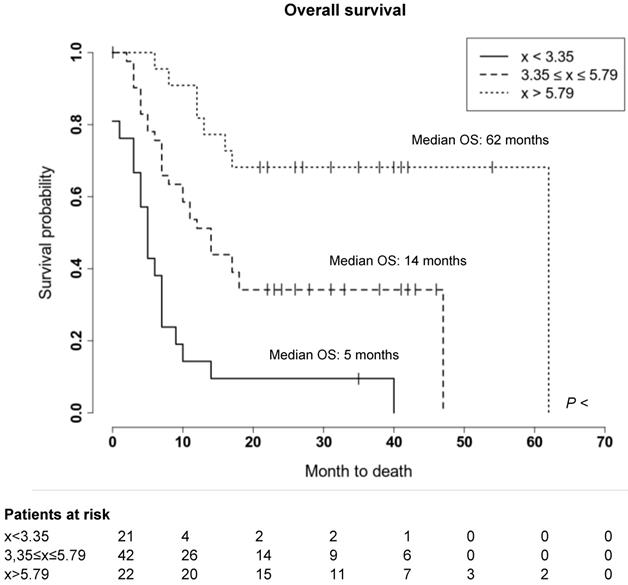
We further evaluated whether the observed wide variability of spleen CXCR4 expression is also associated with clinical outcome of MM patients. Indeed, a significant correlation between splenic uptake and overall survival (OS) could be observed. Thus, we compared OS by stratifying all patients according to the median SUVpeakSpleen into three groups of low, intermediate and high spleen CXCR4 expression (Figure 3). While in the group with a high SUVpeakSpleen >5.79 (TBR >3.51), the OS of MM patients was 62 months, patients in the group with an intermediate SUVpeakSpleen of 3.35 to 5.79 (TBR 2.11-3.50) and in the group with a low SUVpeakSpleen <3.35 (TBR <2.11) showed a significantly lower median OS of 14 and 5 months, respectively (Figure 4, P < 0.001). The association remained significant in multivariate Cox regression analysis (including age, EMD, number of PET-positive focal lesions, anemia, ISS stage, elevated CRP level and number of prior treatment lines), confirming that the splenic 68Ga-Pentixafor signal can provide independent prognostic information in MM (HR 0.75, P = 0.009; Table 3).
In contrast to splenic 68Ga-Pentixafor uptake, mean SUV of the liver was not associated with clinical outcome (Table S1; Figure S1).
Discussion
Here, we present the first study investigating splenic uptake of 68Ga-Pentixafor in MM patients. Previously, we and others have already demonstrated the feasibility of CXCR4-directed PET imaging as a suitable tool for both non-invasive detection of MM lesions as well as patient identification for CXCR4-directed therapy with pilot studies suggesting the feasibility of CXCR4-directed radioligand therapy as a novel treatment approach for MM [29]. In this study, we show for the first time that the intensity of 68Ga-Pentixafor uptake in the spleen, an organ which is frequently overlooked during myeloma examinations, is strongly associated with OS and with parameters that indicate extramedullary hematopoiesis (EMH).
Spleen 68Ga-Pentixafor uptake during MM therapy. Association between splenic 68Ga-Pentixafor uptake at baseline and during disease progression with increasing lines of therapy. A: Representative 68Ga-Pentixafor-PET/CT images for patients with plasma cell disorders with increasing lines of therapies are shown. While the patient with smoldering myeloma (SMM) presents with intense tracer uptake, the splenic signal gradually continues to decrease with increasing lines of therapy.
Results of multivariate cox regression analysis assessing relationship between SUVpeakSpleen, clinical parameters and overall survival
| Variable | Hazard Ratio | 95% Confidence interval | P-value |
|---|---|---|---|
| SUVpeakSpleen | 0.75 | 0.68-0.84 | 0.009* |
| Age | 1.00 | 0.98-1.02 | 0.907 |
| Number of PET-positive lesions | 1.16 | 1.02-1.31 | 0.249 |
| Extramedullary disease | 1.00 | 0.67-1.48 | 0.995 |
| Number of prior treatment lines | 1.09 | 1.03-1.16 | 0.123 |
| Anemia | 1.77 | 1.06-2.98 | 0.269 |
| ISS stage | 1.42 | 1.07-1.89 | 0.215 |
| Elevated CRP level | 0.79 | 0.40-1.56 | 0.730 |
*A P-value <0.05 was considered significant.
Noteworthy, our results are in line with a recent report by the Little Rock group who reported on prognostic implications of the diffusion-weighted magnetic resonance imaging-derived restriction level of the spleen in 295 newly diagnosed MM patients [34]. The authors hypothesized that the spleen signal might prove a promising proxy for tumor load in MM and also be associated with response and prognosis. Since absence of spleen signal was highly correlated with higher myeloma burden in terms of malignant bone marrow plasma cells, they postulated that EMH might be the underlying biologic mechanism for this phenomenon, assuming that MM cells crowd out other hematopoietic cells in the BM. This was questioned by a recent Japanese study of 96 NDMM patients investigating the clinical significance of loss of spleen visualization on DW-MRI. They could also show that loss of spleen visualization is associated with high tumor burden and poor prognosis [35].
Spleen 68Ga-Pentixafor uptake during MM therapy. The association between spleen 68Ga-Pentixafor uptake and therapy lines is depicted (SMM 7.33 ± 1.40; Conn 6.02 ± 1.16; ≤2TL 5.48 ± 1.93; >2TL 4.06 ± 1.43). P-value °<0.05, °°<0.01; °°°<0.001.
Prognostic value of splenic 68Ga-Pentixafor uptake. Stratification of MM patients in three prognostic groups using SUVpeakSpleen: I. <3.35; II. 3.35-5.79; III. >5.79.
Prognostic value of splenic 68Ga-Pentixafor uptake. Overall survival of MM patients stratified by SUVpeakSpleen in three prognostic subgroups. Patients with a SUVpeakSpleen >5.79 showed a significantly longer overall survival (P < 0.001). Given are the cumulated survival (y-axis) and the overall survival (in months; x-axis).
In our cohort of pre-treated MM patients, we were able to corroborate these MRI findings with 68Ga-Pentixafor uptake of the spleen being negatively associated with ISS stage at the time point of PET imaging, percentage of malignant plasma cells in the bone marrow as well as presence of extramedullary disease at the time point of PET imaging. Of note, also disease duration and intensity of previous treatment could be correlated with the splenic PET signal: Whereas untreated patients with SMM demonstrated the highest 68Ga-Pentixafor uptake in the spleen with an SUVpeak of 7.33 ± 1.40 (as compared to controls with an SUVpeakSpleen of 6.02 ± 1.16), tracer accumulation diminished with an increasing number of treatment lines. Since higher blood counts were associated with higher SUVpeakSpleen, one might hypothesize that the spleen might also serve as a biomarker of disease stage. Having stratified patients into a high, intermediate and low CXCR4 expression group, respectively, reduced splenic 68Ga-Pentixafor uptake was significantly associated with shorter OS, independent of the number of prior treatment lines.
The underlying biology of the observed phenomenon is still not elucidated yet as spleen biopsies could not be obtained in the recent study and analysis using autopsy specimens is of limited value given the high autolytic activity within the spleen. One potential explanation is that reduced splenic CXCR4 expression is a marker of reduced EMH. For example, Cao and colleagues observed in an experiment with luciferase-labeled HSCs in mice that the most frequent site of initial engraftment was the spleen and that signal intensity steadily increased over the first 6 weeks after transplant. They proposed that - after myeloablation - the spleen is able to convert from a predominantly lymphoid into a hematopoietic tissue [39, 40]. Additionally, Miwa et al. were able to show that in EMH conditions CXCL12 expression in human spleens is higher than in an environment without EMH [41], supporting our thesis of monitoring hematopoietic constitution via CXCR4-directed imaging.
Another possibility derived from magnetic resonance imaging is that the reduced signal originates from iron overload in the spleen due to red blood cell transfusions. Of note, we were able to record a significant inverse correlation between serum ferritin levels and the splenic 68Ga-Pentixafor uptake, it is thus conceivable that the reduction of the measurable signal might be due to the displacement of CXCR4 positive cells. In another sense, ferritin also acts as an acute-phase protein, thus elevated serum levels might indicate inflammatory activity. Consistent with this, C-reactive protein - another proven adverse prognostic factor in MM [25, 26] - was also found to have a significant inverse correlation with SUVpeak of the spleen in our cohort.
In contrast, the theory that myeloma plasma cells might directly modify the spleen signal by infiltrating this organ is rather unlikely since we did not observe any correlation between myeloma CXCR4 positivity and SUVpeak of the spleen. Since no relevant correlation between overall survival and radiotracer accumulation in the liver could be observed in our cohort, a tumor sink effect as a major contributor to the observed phenomenon seems unlikely. Along these lines, a recent publication in 90 patients with histologically proven solid cancers undergoing CXCR4-targeted PET/CT [42] also reported no relevant tumor sink effect. Further research to gain a better understanding of the biologic processes and cell types responsible for the present observation is highly needed.
Beyond hematologic malignancies, Lewis at al. recently analyzed splenic 68Ga-Pentixafor uptake in 145 patients with various solid cancers [33]. Although platelet counts and/or leukocyte counts correlated positively with spleen 68Ga-Pentixafor uptake in non-small cell lung cancer, small cell lung cancer and neuroendocrine tumors, no association between (increased or decreased) spleen 68Ga-Pentixafor uptake and survival or prior systemic treatment could be observed. Whereas CXCR4 signaling and consequently differences in spleen 68Ga-Pentixafor uptake might be more relevant in MM patients than in solid cancers, no firm conclusions can be drawn yet. Further studies need to clarify the mechanism underlying the highly variable spleen 68Ga-Pentixafor uptake of MM patients to better assess both clinical and therapeutic consequences.
This retrospective study suffers from several limitations. First, we included a rather heterogeneous population of pre-treated MM patients. 84/87 patients received at least one stem cell transplantation, but the number of previous therapy varied to up to 12 different lines of treatment. Secondly, lacking physiologic uptake values of 68Ga-Pentixafor for the spleen in a healthy population, we used the data from a small collective of patients suffering from Conn´s disease to determine decreased and increased uptake. Nonetheless, these values performed well in distinguishing three groups with highly significant different outcomes.
Conclusions
Our data suggest that CXCR4 expression in the spleen might provide prognostic information in pre-treated MM patients. Further research to elucidate the underlying biologic implications is warranted.
Abbreviations
CXCR4: C-X-C motif chemokine receptor 4; MM: multiple myeloma; SUVpeak: standardized uptake values; BM: bone marrow; PET/CT: positron emission tomography/computed tomography; 18F-FDG: 18F-labelled fluorodeoxyglucose; HSCs: hematopoietic stem cells; SDF-1α: stromal derived factor 1; CXCL12: C-X-C motif chemokine ligand 12; DW-MRI: diffusion-weighted magnetic resonance imaging; NDMM: newly diagnosed MM; RBC: red blood cell count; Plt: platelet count; Hb: hemoglobin; Hct: hematocrit; LDH: lactate dehydrogenase; FISH: fluorescence in situ hybridization; SMM: smoldering multiple myeloma; OS: overall survival; EMD: extramedullary disease; VOI: volume of interest; TBR: target-to-background ratio; EMH: extramedullary hematopoiesis.
Supplementary Material
Supplementary figure and tables.
Acknowledgements
We thank the Hematology/Medical Oncology and Nuclear Medicine staff members at the institutions in Würzburg and TU Munich for their support. SK was supported by the IZKF Würzburg. LR was supported by German Cancer Aid via the MSNZ programme and the IZKF Würzburg.
Author Contributions
SK, PK, MK, SH, KMK, UK, LR and CL designed the study. AKB and CL developed the methodology. SK, PK, MK, RT, AG, VAK, PH, AR, LR and CL contributed to data acquisition. AR performed the pathological analysis. SK, PK, MK and CL wrote the manuscript. SK, PK, MK, KMK, NW, AKB, WAW, AD, SH, AG, RAW, AS, UK, LR and CL analyzed and interpreted the data. AKB, HJW, HE, WAW, UK and CL supervised the study. All authors critically reviewed and approved the final manuscript.
Competing Interests
Hans-Jürgen Wester is the founder and shareholder of Scintomics. Ulrich Keller received advisory board fees from Pentixapharm. All other authors have declared that no competing interest exists.
References
1. Rollig C, Knop S, Bornhauser M. Multiple myeloma. Lancet. 2015;385:2197-208
2. Bolli N, Avet-Loiseau H, Wedge DC, Van Loo P, Alexandrov LB, Martincorena I. et al. Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nat Commun. 2014;5:2997
3. Rasche L, Chavan SS, Stephens OW, Patel PH, Tytarenko R, Ashby C. et al. Spatial genomic heterogeneity in multiple myeloma revealed by multi-region sequencing. Nat Commun. 2017;8:268
4. Merz M, Merz AMA, Wang J, Wei L, Hu Q, Hutson N. et al. Deciphering spatial genomic heterogeneity at a single cell resolution in multiple myeloma. Nat Commun. 2022;13:807
5. Terpos E, Dimopoulos MA, Moulopoulos LA. The Role of Imaging in the Treatment of Patients With Multiple Myeloma in 2016. Am Soc Clin Oncol Educ Book. 2016;35:e407-17
6. Cavo M, Terpos E, Nanni C, Moreau P, Lentzsch S, Zweegman S. et al. Role of (18)F-FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: a consensus statement by the International Myeloma Working Group. Lancet Oncol. 2017;18:e206-e17
7. Zamagni E, Patriarca F, Nanni C, Zannetti B, Englaro E, Pezzi A. et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2011;118:5989-95
8. Usmani SZ, Mitchell A, Waheed S, Crowley J, Hoering A, Petty N. et al. Prognostic implications of serial 18-fluoro-deoxyglucose emission tomography in multiple myeloma treated with total therapy 3. Blood. 2013;121:1819-23
9. Davies FE, Rosenthal A, Rasche L, Petty NM, McDonald JE, Ntambi JA. et al. Treatment to suppression of focal lesions on positron emission tomography-computed tomography is a therapeutic goal in newly diagnosed multiple myeloma. Haematologica. 2018;103:1047-53
10. Lapa C, Luckerath K, Malzahn U, Samnick S, Einsele H, Buck AK. et al. 18 FDG-PET/CT for prognostic stratification of patients with multiple myeloma relapse after stem cell transplantation. Oncotarget. 2014;5:7381-91
11. Stolzenburg A, Luckerath K, Samnick S, Speer M, Kneer K, Schmid JS. et al. Prognostic value of [(18)F]FDG-PET/CT in multiple myeloma patients before and after allogeneic hematopoietic cell transplantation. Eur J Nucl Med Mol Imaging. 2018;45:1694-704
12. von Hinten J, Kircher M, Dierks A, Pfob CH, Higuchi T, Pomper MG. et al. Molecular Imaging in Multiple Myeloma—Novel PET Radiotracers Improve Patient Management and Guide Therapy. Front Nucl Med. 2022;2:801792
13. Cassou-Mounat T, Balogova S, Nataf V, Calzada M, Huchet V, Kerrou K. et al. 18F-fluorocholine versus 18F-fluorodeoxyglucose for PET/CT imaging in patients with suspected relapsing or progressive multiple myeloma: a pilot study. Eur J Nucl Med Mol Imaging. 2016;43:1995-2004
14. Dankerl A, Liebisch P, Glatting G, Friesen C, Blumstein NM, Kocot D. et al. Multiple Myeloma: Molecular Imaging with 11C-Methionine PET/CT-Initial Experience. Radiology. 2007;242:498-508
15. Lapa C, Knop S, Schreder M, Rudelius M, Knott M, Jorg G. et al. 11C-Methionine-PET in Multiple Myeloma: Correlation with Clinical Parameters and Bone Marrow Involvement. Theranostics. 2016;6:254-61
16. Lapa C, Garcia-Velloso MJ, Luckerath K, Samnick S, Schreder M, Otero PR. et al. (11)C-Methionine-PET in Multiple Myeloma: A Combined Study from Two Different Institutions. Theranostics. 2017;7:2956-64
17. Ho CL, Chen S, Leung YL, Cheng T, Wong KN, Cheung SK. et al. 11C-acetate PET/CT for metabolic characterization of multiple myeloma: a comparative study with 18F-FDG PET/CT. J Nucl Med. 2014;55:749-52
18. Zlotnik A, Burkhardt AM, Homey B. Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol. 2011;11:597-606
19. Jacobson O, Weiss ID. CXCR4 chemokine receptor overview: biology, pathology and applications in imaging and therapy. Theranostics. 2013;3:1-2
20. Zhao H, Guo L, Zhao H, Zhao J, Weng H, Zhao B. CXCR4 over-expression and survival in cancer: a system review and meta-analysis. Oncotarget. 2015;6:5022-40
21. Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761-7
22. Domanska UM, Kruizinga RC, Nagengast WB, Timmer-Bosscha H, Huls G, de Vries EG. et al. A review on CXCR4/CXCL12 axis in oncology: no place to hide. Eur J Cancer. 2013;49:219-30
23. Kircher M, Herhaus P, Schottelius M, Buck AK, Werner RA, Wester HJ. et al. CXCR4-directed theranostics in oncology and inflammation. Ann Nucl Med. 2018;32:503-11
24. Lapa C, Schreder M, Schirbel A, Samnick S, Kortum KM, Herrmann K. et al. [(68)Ga]Pentixafor-PET/CT for imaging of chemokine receptor CXCR4 expression in multiple myeloma - Comparison to [(18)F]FDG and laboratory values. Theranostics. 2017;7:205-12
25. Lapa C, Hanscheid H, Kircher M, Schirbel A, Wunderlich G, Werner RA. et al. Feasibility of CXCR4-Directed Radioligand Therapy in Advanced Diffuse Large B-Cell Lymphoma. J Nucl Med. 2019;60:60-4
26. Williams SA, Harata-Lee Y, Comerford I, Anderson RL, Smyth MJ, McColl SR. Multiple functions of CXCL12 in a syngeneic model of breast cancer. Mol Cancer. 2010;9:250
27. Ito S, Sato T, Maeta T. Role and Therapeutic Targeting of SDF-1alpha/CXCR4 Axis in Multiple Myeloma. Cancers (Basel). 2021;13:1793
28. Herrmann K, Schottelius M, Lapa C, Osl T, Poschenrieder A, Hanscheid H. et al. First-in-Human Experience of CXCR4-Directed Endoradiotherapy with 177Lu- and 90Y-Labeled Pentixather in Advanced-Stage Multiple Myeloma with Extensive Intra- and Extramedullary Disease. J Nucl Med. 2016;57:248-51
29. Lapa C, Herrmann K, Schirbel A, Hanscheid H, Luckerath K, Schottelius M. et al. CXCR4-directed endoradiotherapy induces high response rates in extramedullary relapsed Multiple Myeloma. Theranostics. 2017;7:1589-97
30. Buck AK, Haug A, Dreher N, Lambertini A, Higuchi T, Lapa C. et al. Imaging of C-X-C Motif Chemokine Receptor 4 Expression in 690 Patients with Solid or Hematologic Neoplasms using (68)Ga-PentixaFor PET. J Nucl Med. 2022 jnumed.121.263693
31. Vag T, Gerngross C, Herhaus P, Eiber M, Philipp-Abbrederis K, Graner FP. et al. First Experience with Chemokine Receptor CXCR4-Targeted PET Imaging of Patients with Solid Cancers. J Nucl Med. 2016;57:741-6
32. Werner RA, Kircher S, Higuchi T, Kircher M, Schirbel A, Wester HJ. et al. CXCR4-Directed Imaging in Solid Tumors. Front Oncol. 2019;9:770
33. Lewis R, Habringer S, Kircher M, Hefter M, Peuker CA, Werner R. et al. Investigation of spleen CXCR4 expression by [(68)Ga]Pentixafor PET in a cohort of 145 solid cancer patients. EJNMMI Res. 2021;11:77
34. Rasche L, Kumar M, Gershner G, Samant R, Van Hemert R, Heidemeier A. et al. Lack of Spleen Signal on Diffusion Weighted MRI is associated with High Tumor Burden and Poor Prognosis in Multiple Myeloma: A Link to Extramedullary Hematopoiesis? Theranostics. 2019;9:4756-63
35. Terao T, Machida Y, Tateishi U, Tsushima T, Narita K, Ikeda D. et al. Association of loss of spleen visualization on whole-body diffusion-weighted imaging with prognosis and tumor burden in patients with multiple myeloma. Sci Rep. 2021;11:23978
36. Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV. et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538-48
37. Rieckmann M, Delgobo M, Gaal C, Buchner L, Steinau P, Reshef D. et al. Myocardial infarction triggers cardioprotective antigen-specific T helper cell responses. J Clin Invest. 2019;129:4922-36
38. Martin R, Juttler S, Muller M, Wester HJ. Cationic eluate pretreatment for automated synthesis of [(6)(8)Ga]CPCR4.2. Nucl Med Biol. 2014;41:84-9
39. Short C, Lim HK, Tan J, O'Neill HC. Targeting the Spleen as an Alternative Site for Hematopoiesis. Bioessays. 2019;41:e1800234
40. Cao YA, Wagers AJ, Beilhack A, Dusich J, Bachmann MH, Negrin RS. et al. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci U S A. 2004;101:221-6
41. Miwa Y, Hayashi T, Suzuki S, Abe S, Onishi I, Kirimura S. et al. Up-regulated expression of CXCL12 in human spleens with extramedullary haematopoiesis. Pathology. 2013;45:408-16
42. Serfling SE, Lapa C, Dreher N, Hartrampf PE, Rowe SP, Higuchi T. et al. Impact of Tumor Burden on Normal Organ Distribution in Patients Imaged with CXCR4-Targeted [(68)Ga]Ga-PentixaFor PET/CT. Mol Imaging Biol. 2022;24:659-65
Author contact
![]() Corresponding author: Constantin Lapa, MD, Nuclear Medicine, Faculty of Medicine, University of Augsburg, Stenglinstr. 2, 86156 Augsburg, Germany. E-mail: Constantin.Lapade; Phone: +49 821-400-2050; Fax: +49 821-400-3057.
Corresponding author: Constantin Lapa, MD, Nuclear Medicine, Faculty of Medicine, University of Augsburg, Stenglinstr. 2, 86156 Augsburg, Germany. E-mail: Constantin.Lapade; Phone: +49 821-400-2050; Fax: +49 821-400-3057.
 Global reach, higher impact
Global reach, higher impact