13.3
Impact Factor
Theranostics 2022; 12(13):6021-6037. doi:10.7150/thno.70448 This issue Cite
Research Paper
Engineered extracellular vesicles with high collagen-binding affinity present superior in situ retention and therapeutic efficacy in tissue repair
1. Department of Surgery, University of California Davis, Sacramento, CA 95817, USA.
2. Institute for Pediatric Regenerative Medicine, Shriners Hospitals for Children Northern California, Sacramento, CA 95817, USA.
3. Department of Radiation Oncology, University of California Davis, Sacramento, CA 95817, USA.
4. Department of Biomedical Engineering, University of California Davis, Davis, CA 95616, USA.
5. Stem Cell Program, Department of Internal Medicine, University of California Davis Medical Center, Sacramento, CA 95817, USA.
6. Department of Biochemistry and Molecular Medicine, University of California Davis, Sacramento, CA 95817, USA.
7. Department of Orthopaedic Surgery, School of Medicine, University of California Davis, Sacramento, CA 95817, USA.
* The authors contributed equally to this work.
Abstract
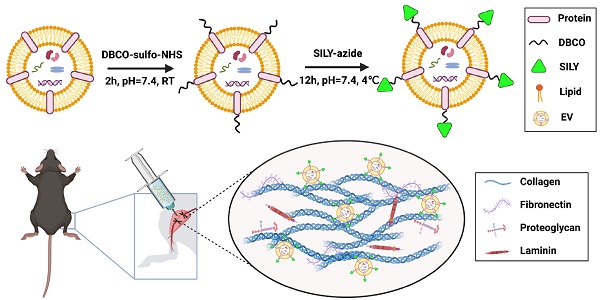
Although stem cell-derived extracellular vesicles (EVs) have remarkable therapeutic potential for various diseases, the therapeutic efficacy of EVs is limited due to their degradation and rapid diffusion after administration, hindering their translational applications. Here, we developed a new generation of collagen-binding EVs, by chemically conjugating a collagen-binding peptide SILY to EVs (SILY-EVs), which were designed to bind to collagen in the extracellular matrix (ECM) and form an EV-ECM complex to improve EVs' in situ retention and therapeutic efficacy after transplantation.
Methods: SILY was conjugated to the surface of mesenchymal stem/stromal cell (MSC)-derived EVs by using click chemistry to construct SILY-EVs. Nanoparticle tracking analysis (NTA), ExoView analysis, cryogenic electron microscopy (cryo-EM) and western-blot analysis were used to characterize the SILY-EVs. Fluorescence imaging (FLI), MTS assay, ELISA and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) were used to evaluate the collagen binding and biological functions of SILY-EVs in vitro. In a mouse hind limb ischemia model, the in vivo imaging system (IVIS), laser doppler perfusion imaging (LDPI), micro-CT, FLI and RT-qPCR were used to determine the SILY-EV retention, inflammatory response, blood perfusion, gene expression, and tissue regeneration.
Results: In vitro, the SILY conjugation significantly enhanced EV adhesion to the collagen surface and did not alter the EVs' biological functions. In the mouse hind limb ischemia model, SILY-EVs presented longer in situ retention, suppressed inflammatory responses, and significantly augmented muscle regeneration and vascularization, compared to the unmodified EVs.
Conclusion: With the broad distribution of collagen in various tissues and organs, SILY-EVs hold promise to improve the therapeutic efficacy of EV-mediated treatment in a wide range of diseases and disorders. Moreover, SILY-EVs possess the potential to functionalize collagen-based biomaterials and deliver therapeutic agents for regenerative medicine applications.
Keywords: Extracellular vesicle, collagen-binding, in situ retention, therapeutic efficacy, tissue repair
 Global reach, higher impact
Global reach, higher impact