13.3
Impact Factor
Theranostics 2022; 12(14):6143-6154. doi:10.7150/thno.75837 This issue Cite
Research Paper
Redox dyshomeostasis modulation of the tumor intracellular environment through a metabolic intervention strategy for enhanced photodynamic therapy
1. Lab of Molecular Imaging and Translational Medicine (MITM), Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, School of Life Science and Technology, Xidian University & International Joint Research Center for Advanced Medical Imaging and Intelligent Diagnosis and Treatment, Xi'an, Shaanxi 710126, China.
2. Academy of Advanced Interdisciplinary Research, Xidian University, Xi'an, Shaanxi, 710071, China.
3. School of Chemical and Biomedical Engineering, Nanyang Technological University, 70 Nanyang Drive, Singapore, 637457, Singapore.
*These authors contributed equally to this work.
Abstract
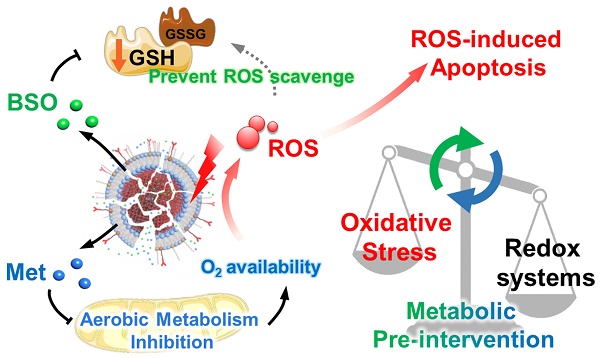
Rationale: Photodynamic therapy (PDT) is a clinically approved anticancer treatment with a promising therapeutic prospect, however, usually suffers from the unfavorable intracellular environment including cellular hypoxia and excessive glutathione (GSH). Comprehensive and long-term modulation of tumor intracellular environment is crucial for optimizing therapeutic outcomes. However, current strategies do not enable such requirements, mainly limited by flexible networks of intracellular metabolic avenues.
Methods: A metabolic pre-intervention (MPI) strategy that targets critical pathways of cellular metabolism, ensuring long-term modulation of the intracellular environment. A versatile lipid-coating photosensitive metal-organic framework (MOF) nano-vehicle encapsulating aerobic respiration inhibitor metformin (Met) and GSH biosynthesis inhibitor buthionine sulfoximine (BSO) (termed PBMLR) was developed for comprehensive sustainable hypoxia alleviation and GSH downregulating.
Results: Since MPI could effectively circumvent the compensatory accessory pathway, PBMLR, therefore functioned as an efficient singlet oxygen (1O2) radical generator during the subsequent laser irradiation process and enhanced PDT anti-tumor efficiency. We emphasized the concordance of long-term hypoxia alleviation, persistent GSH depletion, and tumor enrichment of photosensitizers, which is very meaningful for a broad therapeutic time window and the successful enhancement of PDT.
Conclusion: Our findings indicate that maintaining the sensitivity of tumor cells via MPI could enhance anti-tumor PDT, and may be applied to other dynamic therapies such as radiodynamic therapy and sonodynamic therapy.
Keywords: photodynamic therapy, metal-organic framework, metabolic pre-intervention, hypoxia, glutathione
 Global reach, higher impact
Global reach, higher impact