13.3
Impact Factor
Theranostics 2022; 12(14):6207-6222. doi:10.7150/thno.75323 This issue Cite
Research Paper
Metal-fluorouracil networks with disruption of mitochondrion enhanced ferroptosis for synergistic immune activation
State Key Laboratory of Chemo/Bio-Sensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, P. R. China.
*These authors contributed equally to this work.
Abstract
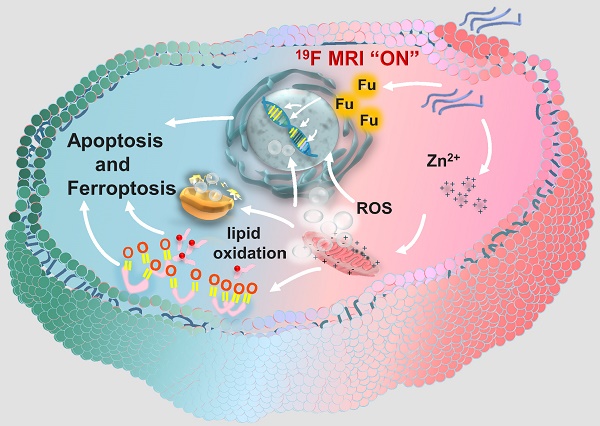
Rationale: Ferroptosis drugs inducing cancer immunogenic cell death (ICD) have shown the potential of immunotherapy in vivo. However, the current ferroptosis drugs usually induce the insufficient immune response because of the low ROS generation efficiency.
Methods: Herein, we design zinc-fluorouracil metallodrug networks (Zn-Fu MNs), by coordinating Zn and Fu via facile one-pot preparation, to inactivate mitochondrial electron transport for enhanced ROS production and immune activation.
Results: Zn-Fu MNs can be responsive toward acidity and adenosine triphosphate (ATP) with the release of Fu and Zn2+, during which Zn2+ can induce mitochondrion disruption to produce ROS, resulting in ferroptosis of cancer cells and 5-Fu interferes with DNA synthesis in nuclei with 19F-MRI signal to be switched on for correlating drug release. With the synergistic effect of DNA damage and ferroptosis, the cancer cells are forced to promote ICD. Thereby, Zn-Fu MNs exhibit the excellent immune response without any other antigens loading. As a result, the infiltration of T cells within tumor and activation of immune cells in spleen have been greatly enhanced.
Conclusions: Combined DNA damage and ferroptosis, Zn-Fu MNs induce the violent emission of tumor associated antigens within cancer cells which will sensitize naive dendritic cells and promote the activation and recruitment of cytotoxic T lymphocytes to exterminate cancer cells. Therefore, the obtained Zn-Fu MNs as ferroptosis inducers can effectively remodel immunosuppressive tumor microenvironment and activate antitumor immune reaction.
Keywords: Immunotherapy, ferroptosis, fluorouracil, 19F MR imaging, cancer theranostics
 Global reach, higher impact
Global reach, higher impact