13.3
Impact Factor
Theranostics 2022; 12(16):7067-7079. doi:10.7150/thno.73509 This issue Cite
Research Paper
Advancing 89Zr-immuno-PET in neuroscience with a bispecific anti-amyloid-beta monoclonal antibody - The choice of chelator is essential
1. Amsterdam UMC location Vrije Universiteit Amsterdam, dept Radiology & Nuclear Medicine, De Boelelaan 1117, Amsterdam, The Netherlands.
2. Amsterdam Neuroscience, Brain imaging, Amsterdam, The Netherlands.
3. H. Lundbeck A/S, Ottiliavej 9, 2500 Valby, Denmark
Abstract
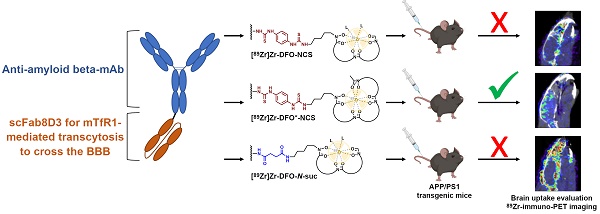
The accelerated approval of the monoclonal antibody (mAb) aducanumab as a treatment option for Alzheimer's Disease and the continued discussions about its efficacy have shown that a better understanding of immunotherapy for the treatment of neurodegenerative diseases is needed. 89Zr-immuno-PET could be a suitable tool to open new avenues for the diagnosis of CNS disorders, monitoring disease progression, and assessment of novel therapeutics. Herein, three different 89Zr-labeling strategies and direct radioiodination with 125I of a bispecific anti-amyloid-beta aducanumab derivate, consisting of aducanumab with a C-terminal fused anti-transferrin receptor binding single chain Fab fragment derived from 8D3 (Adu-8D3), were compared ex vivo and in vivo with regard to brain uptake and target engagement in an APP/PS1 Alzheimer's disease mouse model and wild type animals.
Methods: Adu-8D3 and a negative control antibody, based on the HIV specific B12 antibody also carrying C-terminal fused 8D3 scFab (B12-8D3), were each conjugated with NCS-DFO, NCS-DFO*, or TFP-N-suc-DFO-Fe-ester, followed by radiolabeling with 89Zr. 125I was used as a substitute for 124I for labeling of both antibodies. 30 µg of radiolabeled mAb, corresponding to approximately 6 MBq 89Zr or 2.5 MBq 125I, were injected per mouse. PET imaging was performed 1, 3 and 7 days post injection (p.i.). All mice were sacrificed on day 7 p.i. and subjected to ex vivo biodistribution and brain autoradiography. Immunostaining on brain tissue was performed after autoradiography for further validation.
Results: Ex vivo biodistribution revealed that the brain uptake of [89Zr]Zr-DFO*-NCS-Adu-8D3 (2.19 ±0.12 %ID/g) was as high as for its 125I-analog (2.21 ±0.15 %ID/g). [89Zr]Zr-DFO-NCS-Adu-8D3 and [89Zr]Zr-DFO-N-suc-Adu-8D3 showed significantly lower uptake (< 0.65 %ID/g), being in the same range as for the 89Zr-labeled controls (B12-8D3). Autoradiography of [89Zr]Zr-DFO*-NCS-Adu-8D3 and [125I]I-Adu-8D3 showed an amyloid-beta related granular uptake pattern of radioactivity. In contrast, the [89Zr]Zr-DFO-conjugates and the control antibody groups did not show any amyloid-beta related uptake pattern, indicating that DFO is inferior for 89Zr-immuno-PET imaging of the brain in comparison to DFO* for Adu-8D3. This was confirmed by day 7 PET images showing only amyloid-beta related brain uptake for [89Zr]Zr-DFO*-NCS-Adu-8D3. In wild type animals, such an uptake was not observed. Immunostaining showed a co-localization of all administered Adu-8D3 conjugates with amyloid-beta plaques.
Conclusion: We successfully demonstrated that 89Zr-immuno-PET is suitable for imaging and quantifying amyloid-beta specific brain uptake using a bispecific aducanumab brain shuttling antibody, Adu-8D3, but only when using the novel chelator DFO*, and not DFO, for labeling with 89Zr.
Keywords: Aducanumab, Amyloid-beta, Transferrin receptor, 89Zr-immuno-PET, DFO*
 Global reach, higher impact
Global reach, higher impact