13.3
Impact Factor
Theranostics 2023; 13(1):77-94. doi:10.7150/thno.77404 This issue Cite
Research Paper
DNA hypermethylation-induced miR-182 silence targets BCL2 and HOXA9 to facilitate the self-renewal of leukemia stem cell, accelerate acute myeloid leukemia progression, and determine the sensitivity of BCL2 inhibitor venetoclax
1. Medical Research Center, The First Affiliated Hospital of Wenzhou Medical University, 1 Xuefubei Street, Ouhai District, Wenzhou, Zhejiang Province, China.
2. Department of Clinical Laboratory, The First Affiliated Hospital of Wenzhou Medical University, 1 Xuefubei Street, Ouhai District, Wenzhou, Zhejiang Province, China.
3. The College of Medical Technology, Shanghai University of Medicine& Health Sciences; 279 ZhouZhuGong Street, Pudong District, Shanghai, China.
4. Department of Hematology, The First Affiliated Hospital of Zhejiang Chinese Medical University, 54 Post Road, Hangzhou, Zhejiang Province, China.
5. Department of Hematology, The First Affiliated Hospital of Wenzhou Medical University, 1 Xuefubei Street, Ouhai District, Wenzhou, Zhejiang Province, China.
*These authors contributed equally to this work.
Abstract
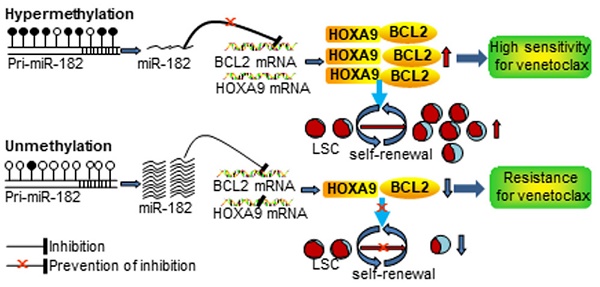
Rationale: microRNAs (miRNAs) are frequently deregulated and play important roles in the pathogenesis and progression of acute myeloid leukemia (AML). miR-182 functions as an onco-miRNA or tumor suppressor miRNA in the context of different cancers. However, whether miR-182 affects the self-renewal of leukemia stem cells (LSCs) and normal hematopoietic stem progenitor cells (HSPCs) is unknown.
Methods: Bisulfite sequencing was used to analyze the methylation status at pri-miR-182 promoter. Lineage-negative HSPCs were isolated from miR-182 knockout (182KO) and wild-type (182WT) mice to construct MLL-AF9-transformed AML model. The effects of miR-182 depletion on the overall survival and function of LSC were analyzed in this mouse model in vivo.
Results: miR-182-5p (miR-182) expression was lower in AML blasts than normal controls (NCs) with hypermethylation observed at putative pri-miR-182 promoter in AML blasts but unmethylation in NCs. Overexpression of miR-182 inhibited proliferation, reduced colony formation, and induced apoptosis in leukemic cells. In addition, depletion of miR-182 accelerated the development and shortened the overall survival (OS) in MLL-AF9-transformed murine AML through increasing LSC frequency and self-renewal ability. Consistently, overexpression of miR-182 attenuated AML development and extended the OS in the murine AML model. Most importantly, miR-182 was likely dispensable for normal hematopoiesis. Mechanistically, we identified BCL2 and HOXA9 as two key targets of miR-182 in this context. Most importantly, AML patients with miR-182 unmethylation had high expression of miR-182 followed by low protein expression of BCL2 and resistance to BCL2 inhibitor venetoclax (Ven) in vitro.
Conclusions: Our results suggest that miR-182 is a potential therapeutic target for AML patients through attenuating the self-renewal of LSC but not HSPC. miR-182 promoter methylation could determine the sensitivity of Ven treatment and provide a potential biomarker for it.
Keywords: Hypermethylation, MicroRNA, Leukemia stem cell, Venetoclax
 Global reach, higher impact
Global reach, higher impact