13.3
Impact Factor
Theranostics 2023; 13(12):4166-4181. doi:10.7150/thno.85843 This issue Cite
Research Paper
Caveolin-mediated cytosolic delivery of spike nanoparticle enhances antitumor immunity of neoantigen vaccine for hepatocellular carcinoma
1. The United Innovation of Mengchao Hepatobiliary Technology Key Laboratory of Fujian Province, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou 350025, P. R. China.
2. Liver Disease Center, The First Affiliated Hospital of Fujian Medical University, Fuzhou 350005, People's Republic of China.
3. CAS Key Laboratory of Design and Assembly of Functional Nanostructures, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, P. R. China.
4. Mengchao Med-X Center, Fuzhou University, Fuzhou 350116, P. R. China.
5. Fujian Agriculture and Forestry University, Fuzhou 350002, P. R. China.
#These authors contributed equally to this paper
Abstract
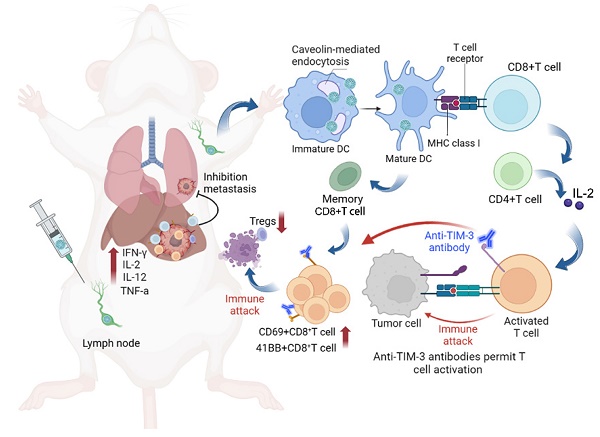
Rationale: Although neoantigen-based cancer vaccines have shown promise in various solid tumors, limited immune responses and clinical outcomes have been reported in patients with advanced disease. Cytosolic transport of neoantigen and adjuvant is required for the activation of intracellular Toll-like receptors (TLRs) and cross-presentation to prime neoantigen-specific CD8+T cells but remains a significant challenge.
Methods: In this study, we aimed to develop a virus-like silicon vaccine (V-scVLPs) with a unique spike topological structure, capable of efficiently co-delivering a hepatocellular carcinoma (HCC)-specific neoantigen and a TLR9 agonist to dendritic cells (DCs) to induce a robust CD8+T cell response to prevent orthotopic tumor growth. We evaluated the antitumor efficacy of V-scVLPs by examining tumor growth and survival time in animal models, as well as analyzing tumor-infiltrating CD8+T cells and cytokine responses in the tumor microenvironment (TME). To evaluate the synergistic efficacy of V-scVLPs in combination with α-TIM-3 in HCC, we used an orthotopic HCC mouse model, a lung metastasis model, and a tumor rechallenge model after hepatectomy.
Results: We found that V-scVLPs can efficiently co-deliver the hepatocellular carcinoma (HCC)-specific neoantigen and the TLR9 agonist to DCs via caveolin-mediated endocytosis. This advanced delivery strategy results in efficient lymph node draining of V-scVLPs to activate lymphoid DC maturation for promoting robust CD8+T cells and central memory T cells responses, which effectively prevents orthotopic HCC tumor growth. However, in the established orthotopic liver tumor models, the inhibitory receptor of TIM-3 was significantly upregulated in tumor-infiltrating CD8+T cells after immunization with V-scVLPs. Blocking the TIM-3 signaling further restored the antitumor activity of V-scVLPs-induced CD8+T cells, reduced the proportion of regulatory T cells, and increased the levels of cytokines to alter the tumor microenvironment to efficiently suppress established orthotopic HCC tumor growth, and inhibit lung metastasis as well as recurrence after hepatectomy.
Conclusion: Overall, the developed novel spike nanoparticles with efficient neoantigen and adjuvant intracellular delivery capability holds great promise for future clinical translation to improve HCC immunotherapy.
Keywords: Virus-like silicon nanoparticles, neoantigen, caveolin, immunotherapy, T cell immunoglobulin and mucin domain 3
 Global reach, higher impact
Global reach, higher impact