13.3
Impact Factor
Theranostics 2023; 13(15):5290-5304. doi:10.7150/thno.84645 This issue Cite
Research Paper
1α,25(OH)2D3 ameliorates insulin resistance by alleviating γδ T cell inflammation via enhancing fructose-1,6-bisphosphatase 1 expression
1. Guangdong Provincial Key Laboratory of Tumor Interventional Diagnosis and Treatment, Zhuhai Institute of Translational Medicine, Zhuhai People's Hospital Affiliated with Jinan University, Jinan University, Zhuhai, 519000, Guangdong, China.
2. The Biomedical Translational Research Institute, Faculty of Medical Science, Jinan University, Guangzhou, 510632, Guangdong, China.
3. Department of Geriatrics, The Seventh Affiliated Hospital, Sun Yat-Sen University, Shenzhen, 518107, Guangdong, China.
4. Department of Endocrinology, Guangdong Second Provincial General Hospital, Guangzhou, 510310, Guangdong, China.
5. Anhui Provincial Center for Disease Control and Prevention, Hefei, 230601, Anhui, China.
6. Department of Oncology, First Affiliated Hospital, Jinan University, Guangzhou, 510632, Guangdong, China.
#These authors contributed equally: Peng Li, Ke Li, and Wenhui Yuan.
*These authors jointly supervised this work.
Abstract
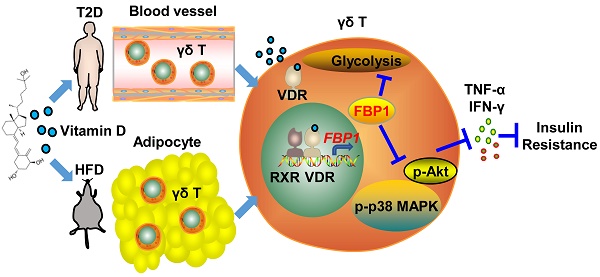
Background: Chronic inflammation caused by immune cells is the central link between obesity and insulin resistance. Targeting the inflammatory process is a highly promising method for reversing systemic insulin resistance.
Methods: Blood samples were prospectively collected from 68 patients with type 2 diabetes. C57BL/6J mice were fed either a high-fat diet (HFD) or normal chow (NC). We performed phenotypical and functional analyses of immune cells using flow cytometry. Vitamin D receptor (VDR) knockout γδ T cells were constructed using Cas9-gRNA targeted approaches to identify 1α,25(OH)2D3/VDR signaling pathway-mediated transcriptional regulation of fructose-1,6-bisphosphatase (FBP1) in γδ T cells.
Results: Serum vitamin D deficiency aggravates inflammation in circulating γδ T cells in type 2 diabetes patients. We defined a critical role for 1α,25(OH)2D3 in regulating glycolysis metabolism, protecting against inflammation, and alleviating insulin resistance. Mechanistically, 1α,25(OH)2D3-VDR promoted FBP1 expression to suppress glycolysis in γδ T cells, thereby inhibiting Akt/p38 MAPK phosphorylation and reducing inflammatory cytokine production. Notably, therapeutic administration of 1α,25(OH)2D3 restrained inflammation in γδ T cells and ameliorated systemic insulin resistance in obese mice.
Conclusions: Collectively, these findings show that 1α,25(OH)2D3 plays an important role in maintaining γδ T cell homeostasis by orchestrating metabolic programs, and is a highly promising target for preventing obesity, inflammation, and insulin resistance.
Keywords: vitamin 1α, 25(OH)2D3, γδ T cell, fructose-1, 6-bisphosphatase 1, glycolysis, inflammation, insulin resistance
 Global reach, higher impact
Global reach, higher impact