13.3
Impact Factor
Theranostics 2023; 13(15):5322-5347. doi:10.7150/thno.87356 This issue Cite
Review
Peptide-drug co-assembling: A potent armament against cancer
1. School of Pharmacy, Henan University of Traditional Chinese Medicine, Zhengzhou 450046, China.
2. Academy of Chinese Medicine Science, Henan University of Traditional Chinese Medicine, Zhengzhou 450046, China.
Received 2023-6-20; Accepted 2023-9-19; Published 2023-9-25
Abstract
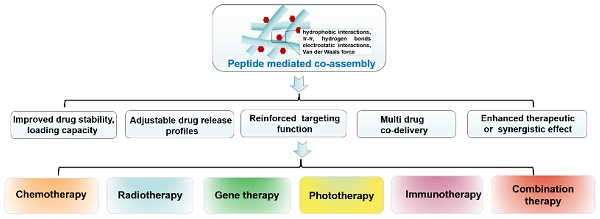
Cancer is still one of the major problems threatening human health and the therapeutical efficacies of available treatment choices are often rather low. Due to their favorable biocompatibility, simplicity of modification, and improved therapeutic efficacy, peptide-based self-assembled delivery systems have undergone significant evolution. Physical encapsulation and covalent conjugation are two common approaches to load drugs for peptide assembly-based delivery, which are always associated with drug leaks in the blood circulation system or changed pharmacological activities, respectively. To overcome these difficulties, a more elegant peptide-based assembly strategy is desired. Notably, peptide-mediated co-assembly with drug molecules provides a new method for constructing nanomaterials with improved versatility and structural stability. The co-assembly strategy can be used to design various nanostructures for cancer therapy, such as nanotubes, nanofibrils, hydrogels, and nanovesicles. Recently, these co-assembled nanostructures have gained tremendous attention for their unique superiorities in tumor therapy. This article describes the classification of assembled peptides, driving forces for co-assembly, and specifically, the design methodologies for various drug molecules in co-assembly. It also highlights recent research on peptide-mediated co-assembled delivery systems for cancer therapy. Finally, it summarizes the pros and cons of co-assembly in cancer therapy and offers some suggestions for conquering the challenges in this field.
Keywords: peptide, co-assembly, nanostructures, drug delivery, cancer therapy
1. Introduction
Cancer is still one of the main problems threatening human health while its available treatment method, such as chemotherapy, radiotherapy, immunotherapy and phototherapy are unsatisfactory and have major side effects [1, 2]. Even though there have been significant advances in cancer treatment, it is still not yet possible to entirely eradicate the disease. With the development of nanomedicine, new nano drug delivery systems, such as nanotubes, nanofibers, micelles and nanoparticles, have made significant progress in enhancing the therapeutic effects, reducing the side effects, and increasing the solubility of anticancer drugs [3, 4]. Although the use of nano drug delivery systems has improved, there are still problems that need to be fixed. For instance, synthetic inorganic materials may be associated with poor biocompatibility and unavoidable immunological responses [5]. Besides, the application of synthetic polymers is limited by the complex functionalization methods and bounded capacity to deliver protein drugs [6]. In addition, due to the complexity of the tumor microenvironment, the single targeting capability of nanodrugs cannot satisfy the requirement for precise drug delivery [7]. Therefore, it is imperative to create a delivery system that is both non-toxic and highly efficient, as well as possessing superior targeting capabilities.
Three types of peptide-based assembly delivery system: (I) Drug-peptide conjugates; (II) Peptide mediated co-assembly; (III) Physical encapsulation.
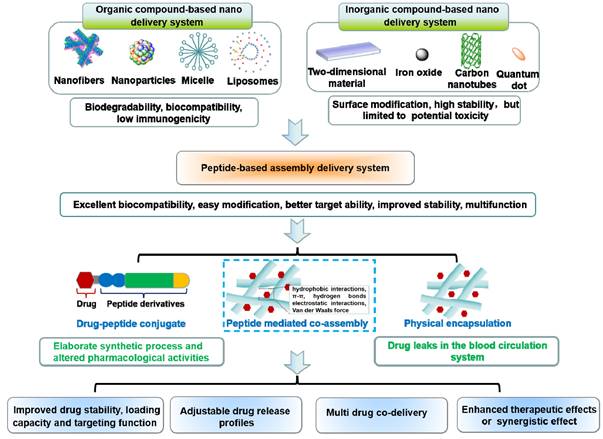
Peptides are a class of compounds containing two or more amino acids linked by amide bonds, which widely exist in the human body and exerts specific biological functions. Peptide self-assembly involves non-covalent interactions that facilitate the spontaneous formation of well-ordered architectures [8, 9]. The superior performance of peptide-based self-assembly systems has garnered considerable attention, with notable advantages such as excellent biocompatibility, simple modification, better scalability, and enhanced stability [10, 11]. Self-assembling peptides themselves exhibits excellent anti-cancer and antibacterial properties [12-14]. Moreover, self-assembling peptides have been exploited as drug delivery carrier for multiple biological applications, such as anti-bacterial, anti-inflammatory and tissue engineering [15-17]. In particular, self-assembled peptide drug delivery holds great promise for treating cancer due to its multifunctionality [18]. In general, physical encapsulation or covalent conjugation can be used to load medicinal molecules into peptides. However, the therapeutic effects of physical encapsulation may be undesirable due to drug leaks in the blood circulation system, and covalent conjugation is challenged by the elaborate synthetic process and altered pharmacological activities. The synthesis of peptide-drug conjugates requires not only solid-phase synthesis, but also complex liquid phase synthesis, as well as subsequent purification steps [19-22]. Besides, even minute adjustments in the structure of peptide-drug covalent conjugates can pose a significant impact on the performance of the system. In ideal conditions, pharmacological activity is increased, but sometimes the opposite results are also seen (Figure 1) [23-24]. To solve these problems, a more elegant peptide-based assembly strategy should be developed.
Notably, peptide-mediated co-assembly with drug molecules provides a new method for constructing nanomaterials with structural complexity and stability. Stable co-assembled supramolecular nanostructures are made possible by the noncovalent interactions, which often include hydrogen bonds, π-π stacking, electrostatic interactions, hydrophobic interactions, and van der Waals forces [25, 26]. The co-assembly strategy can be used to design various nanostructures for cancer therapy, such as nanotubes, nanofibrils, hydrogels, and nanovesicles. Recently, co-assembled nanostructures have gained tremendous attention due to their exceptional advantages in cancer treatment [27-29]. Compared with other delivery systems, the advantages of theses co-assembled nanostructures includes the following aspects: (1) In contrast to simple encapsulation, the stability of the drug can be improved and drug leakage can be avoided by the intermolecular interaction between peptide and drug molecules; (2) Adjustable drug release profiles can be obtained by changing the ration of peptide and drug molecules; (3) The drug loading capacity can be significantly improved through the non-covalent interaction between the residues of peptide and drug molecules; (4) Therapeutic effects of the drug can be enhanced by utilizing multifunctional peptide with excellent targetability; (5) Multiple drug molecule can be simultaneously loaded in co-assembled nanostructures for combination therapy (Figure 1). Comprehensively, these superiorities provide a wide latitude for the development of peptide-based co-assembled nanostructures for cancer therapy.
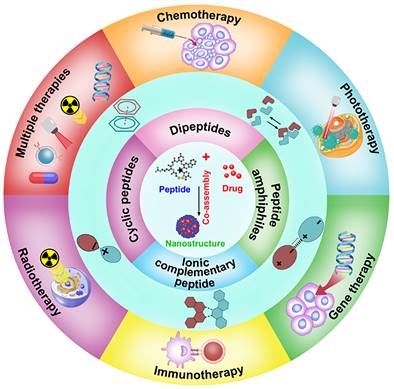
In this review, we summarized the recent research progress on the co-assembled peptides-drug molecule for cancer therapy, including the classification of assembled peptides, driving forces for co-assembly, and specifically, the recent progress in the co-assembly of peptides and drug molecule (Figure 2). We anticipate encouraging opportunities for developing intricate peptide-drug co-assembled nanostructures that offer effectivecancer therapies and support the comprehension and clinical implementation of these nanostructures through this review.
2. Classification of building blocks of assembled peptides
Peptides are compounds with long chain structures composed of polar or non-polar amino acids. According to the various constituent amino acids and different motifs, the building blocks of assembled peptides used for co-assembly can be divided into aromatic dipeptides, peptide amphiphiles, ion complementary peptides and cyclic peptides.
2.1. Aromatic dipeptides
Phenylalanine is the simplest building block commonly used in the construction of self-assembled peptides. Phenylalanine dipeptide (FF) is the core recognition sequence present in Alzheimer's amyloid β-peptide. Phenylalanine dipeptide and its derivatives are always used to build supramolecular materials with various structures via π-π stacking and hydrogen bonding interaction [30]. Gazit and his colleagues revealed that FF self-assemble to form 1D nanotubes with specific structures, which obtained extensive attention in the field of supramolecular peptide-based self-assembly [31, 32]. Afterward, Ulijn and his colleagues carried out systematic research on tripeptides-based self-assembly by utilizing computer simulation experiments [33]. Afterwards, various experiments have demonstrated the morphology of peptide-based self-assembled nanostructure can change from 1D (fiber and tube) to 2D (sheet) by adjusting the sequence of dipeptides derivatives.
Schematic illustration of co-assembled peptide-drug nanomaterials in various cancer therapeutics.
Structure diagram of various building blocks of assembled peptides. (A) Structure diagram of FF-based peptide derivative. (B) Three types of peptide amphiphiles: Amphiphilic peptides, lipidated peptide amphiphiles, and supramolecular PAs. (C) Chemical structures of ionic-complementary peptide RADA16. (D) Assembly process of cyclic peptide.
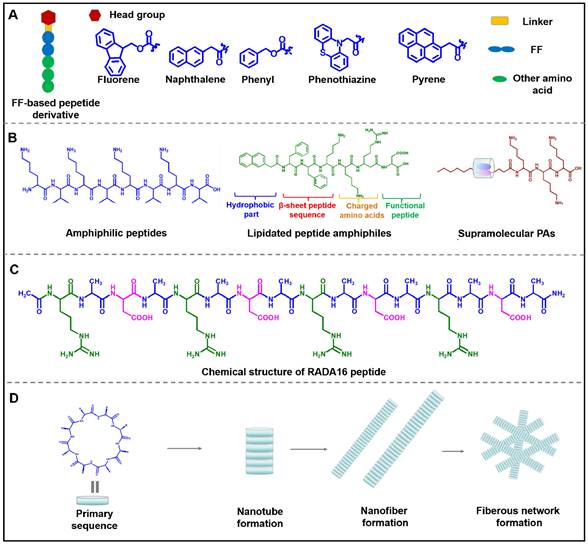
In recent studies with diphenylalanine, it has been demonstrated that FF-based self-assembled nanomaterials exhibited enhanced stability, structural flexibility and accessibility. Besides, the modification of the functional group at the N-terminal or C-terminal of the peptide can not only change the assembly behavior of the peptide, but also endow the peptide with multifunction. The N-terminal dipeptide could be modified by introducing aromatic groups such as naphthylacetic acid (Nap), tert-butoxycarbonyl (Boc), and 9-Fluorenylmethoxycarbonyl (Fmoc) (Figure 3A) [34-36]. Duan et al. designed and synthesized D-Nap-GFFY-T317, which could be self-assembled into nanofibers to effectively inhibit the proliferation of tumor cells [37]. As the research deepens, FF and its derivatives are expected to be employed as multifunctional nanomaterials with varied performance. For example, carboxybenzyl-protected diphenylalanine (ZFF) could co-assemble with different bipyridine derivatives to form various supramolecular nanostructures through intermolecular hydrogen bonding [38]. Besides, different functional groups, such as targeting sequence, specific stimuli-responsive sequence and therapeutic sequence, can be introduced into the C-terminal of the peptide to construct multi-functional nanostructures. Overall, FF is the most favorable peptide building block due to its structural simplicity, functional diversity, and cost-effectiveness. Various supramolecular structures such as nanofiber, nanotubes and ribbons can be obtained by utilizing the self-assembly of FF via the interactions of hydrogen bonding and π-π stacking [39-44]. Many research groups at home and abroad have also successively studied FF-based self-assembly and explored its potential applications in drug delivery, tissue engineering, and antibacterial [45-49]. With the deepening research of FF-based assembly, it can be expected that introducing multifunctional modules into peptides will provide an effective way to obtain nanomaterials with improved intrinsic properties and sophisticated functions, which ultimately realize the potential applications in nanomedicine.
2.2. Peptide amphiphiles
Peptide amphiphiles (PA) are amphiphilic peptides that prefer to form highly defined nanostructures in a particular situation. Peptide amphiphiles can usually be classified into three types: (1) Amphiphilic peptides; (2) Lipidated peptide amphiphiles; (3) Supramolecular PAs [50-53]. Generally, PA can self-assemble into varied nanostructures such as nanofibers, nanotubes and micelles under the balanced intramolecular and intermolecular interactions of amino acid residues. The amphiphilic characteristics inherited by PA enable it assemble into nanostructures with a hydrophobic core and highly hydrated and charged surface. Drug molecules can be loaded in the different parts of PA-based assembled nanostructures. In general, lipophilic drugs molecules can be loaded in the hydrophobic part, while water-solubility drugs molecules usually are encapsulated in the hydrophilic surface.
Amphiphilic peptides are a representative class of PA, composed of pure polar and non-polar amino acids. For example, V6K3 is a positively charged amphiphilic peptide, which exhibited a morphological transformation from nanoparticles to nanofibers in the presence of plasma amine oxidase and could be exploited as an ideal carrier for delivery lipophilic anticancer drugs [54]. Among all PA, lipidated peptide amphiphiles are the most representatives PA, which usually consists of four essential domains as shown in Figure 3B: (1) hydrophobic domain, such as alkyl chain, fluorene methoxycarbonyl (Fmoc) or 2-naphthylacetic acid (Nap); (2) β-sheet peptide sequence for promoting peptide self-assembly; (3) Negatively charged or positively amino acids, which endow peptide with a certain solubility in water; (4) functional peptide, such as cell adhesion peptides and targeting peptide [55, 56]. During the process of self-assembly of lipidated peptide amphiphiles, the hydrophobic domain can form internal hydrophobic nuclei, while the functional part is exposed to the surface of the nanostructure. β-sheet sequence and charged amino acids paly an indispensable role in regulating the stability and solubility of peptides. Lipidated peptide amphiphiles tend to form 3D nanofibers, and then the fibers gradually elongate to form hydrogels. Lipidated peptide amphiphiles have demonstrated considerable application prospects in the fields of drug delivery, anti-bacterial therapies, and regenerative medicine thanks to their superior ability to form structurally ordered nanostructures. Supramolecular PAs are obtained by integrating supramolecular chemistry during the preparation process. In brief, two separate molecules are linked by covalent bonds or supramolecular interactions. Debapratim et al designed a ternary complex by using naphthalene functionalized peptide (Nap-P), cucurbit[8]uril (CB[8]) and a viologen unit (MV-CHO). The ternary complex formed a transient supramolecular PA when dodecylamine (DA) exists under basic conditions [57, 58]. Thus, various supramolecular PA with structural flexibility and stimulus responsiveness can be obtained by using this approach. In general, although amphiphilic peptides-based nanomaterials have the virtue of easy preparation, structural flexibility and biocompatibility, amphiphilic peptides-based complicated structures need to be exploited for developing advanced nanomaterials.
2.3 Ionic-Complementary peptide
Ion complementary peptide is another common building block for developing supramolecular nanomaterials, which contains an alternating arrangement of charged amino acid and hydrophobic amino acids residues [59, 60]. The assembly of this peptide relies on the formation of hydrogen bonds, which tend to form β-sheet structures with two different polar and nonpolar surfaces. The polar surface of the β-sheet structure is composed of positively and negatively charged amino acids, and the non-polar surface contains hydrophobic amino acids. The structure of alternate amino acid residues boosts the process of assembly, inducing the formation of the hydrophobic core and hydrophilic surface of nanofiber when the two β-sheet are stacked into a basic fibril unit.
RADA16 is a representative ionic-complementary peptide, which is composed of 16 amino acids, including positively charged arginine (R), hydrophobic alanine (A), and negatively charged aspartic acid (D) (Figure 3C) [61, 62]. More and more research indicated that the modification of RADA16 could endow this peptide with novel functions. Tao et al. designed two functionalized peptides, namely CRP ((RADA)4-GGQQLK) and CBP ((RADA)4-GSVLGYIQIR). These two peptides were regarded as Ca2+ binding peptides, which could self-assemble into nanofibers and crosswise link to form meshlike networks when being exposed to Ca2+. Then, blood coagulation factor XIIIa could further trigger the network to form a compact nano-band-aid to stop bleeding [63]. By conjugating HS-RADA16-GG-melittin and 4-arm PEG-Maleimide, Jin et al. synthesized MRP, which was used to encapsulate the ultrafiltration retentate of irradiated tumor cells (UF) to obtain UF@MRP for synergistically killing tumor cells [64]. Later results showed that RADA16 was capable to form nanofibrous hydrogel for delivering exogenous DCs, antigens and anti-PD-1 antibodies for immunotherapy against cancer [65]. In generals, RADA16 and its derivates can self-assemble into nanofibers, which exhibit good biocompatibility and negligible immunogenicity. The further study focuses on the RADA16 derivates with longer sequences that will promote the development of ionic-complementary peptide-based nanostructures with more flexible structures and stronger mechanical properties.
2.4. Cyclic peptides
In 1974, De Santis et al. profoundly summarized that cyclic peptide consisted of a certain number of alternate D and L-amino acids, which could self-assemble into rigid cylindrical nanotubes with β-sheet structure under non-covalent interaction (Figure 3D) [66]. In the 1990s, Ghadiri and his colleagues designed cyclic peptides and first reported the self-assembled nanotube structure of the cyclic peptide [67]. Follow-up released research comprehensively studied the properties of cyclic peptides [68-70]. Compared with linear peptides, cyclic peptides exhibited several advantages:(1) High stability and reduced degradation of proteases; (2) Simplicity of transmembrane transport. Given the favorable properties of cyclic peptides, many artificial cyclic peptides have been regarded as therapeutic agents and drug delivery carriers. For example, Gao and co-works designed a novel cyclic peptide C25, which could target the immune checkpoint by blocking the interaction of LAG-3 and HLA-DR, leading to promising anticancer effects [71]. Niu and co-works designed a glutathione (GSH)-responsive cyclic peptide (C-1), which could convert into a linear peptide under the overexpressed GSH in cell and further self-assembled into nanofibers, leading to caspase-3 activation and cell death [72]. Schmuck et al. reported a cyclic peptide which was conjugated with guanidiniocarbonyl pyrrole (GCP) residue. The GCP-containing cyclic peptide could assembled into stable nanostructure for delivering DNA into cells [73]. Functionalized cyclic peptides also can be used for enhancing the targeting ability of drugs. Gellerman et al. designed a novel somatostatin-derived cyclic peptides-drug conjugates, which exhibited preferable targetability towards pancreatic cancer cells with overexpressed somatostatin receptors [74]. All in all, thanks to the unique advantages of cyclic peptides, such as versatility, targetability and structural stability, it has been developed as an efficient carrier for the delivery of small-molecule and macromolecule therapeutic agents.
3. Driving forces for co-assembly
Multiple nanomaterials can be designed through the co-assembly of peptide and drug molecules, which exhibit considerable advantages, such as enhanced stability and prolonged retention time. The co-assembly process relies on the balance of intermolecular noncovalent force between peptide and drug molecules, including hydrophobic interactions, aromatic interactions, electrostatic interactions, hydrogen bonds, and Van der Waals force (Figure 4) [75, 76].
3.1. Hydrophobic interactions
Hydrophobic interactions is one of the main driving forces for assembly, which are generate by the hydrophobic amino acids residues in hydrophobic conditions. Amphiphilic molecules in water tend to form micelles rather than nanofibers when the hydrophobic interactions are the only self-assembly force [77, 78]. Hydrophobic parts of amphiphilic molecules are prone to gather to form inner cores when the hydrophilic parts are exposed to water. Significantly, this hydrophobic interaction is essentially ascribed to the entropy effect, not the enthalpy effect. Most notably, there are always several driving forces at work in a co-assembly system, and the main dominant interactions are always associated with the peptide residues and the drug molecule structure. For instance, Chen et al. designed an ionic-complementary peptide EAR8-II, which could co-assemble with liposoluble anticancer drug pirarubicin (THP) into stable EAR8-II-THP nanofiber in aqueous media [79]. Further results indicated that the hydrophobic interactions between aromatic rings of THP and alanine residues in EAR8-II were the dominant forces for the formation of the co-assembled EAR8-II-THP nanocomplexes. Thus, the construction of a nanoplatform by utilizing the hydrophobic interaction between peptide and drug molecules may be a feasible strategy for hydrophobic drugs delivery.
3.2 Aromatic interactions
π-π stacking is one of the main forces of self-assembly and co-assembly of peptides, especially for peptides containing aromatic groups. As a result of the peptides with aromatic groups' low solubility, π-π stacking is quite noticeable in water. When the assembly occurs in organic solvents, the aromatic interactions will function as the unique driving force. Domestic and foreign research groups represented by Gazit et al. have demonstrated that diphenylalanine peptide (L-Phe-L-Phe) could self-assemble into an ordered tubular structure under the π-π interaction and hydrogen bond [80]. Besides, for aromatic groups-containing peptides and drugs, π-π stacking would be the main force for promoting and stabilizing the co-assembled peptide-drug complexes. For example, Nilsson et al. designed several Fmoc-Phe-DAP molecules, which were composed of Fmoc-Phe and diaminopropane. The Fmoc-Phe-DAP could self-assemble into hydrogels under the impact of sodium chloride, which was suitable for delivering diclofenac. In vitro release experiments revealed that the attractive π-π interactions between peptide and diclofenac played an important role in the release amount of diclofenac [81]. Based on the research, thanks to the stable π-π interactions, aromatic peptides would be considerable building blocks for constructing nanoplatforms for delivering drugs containing aromatic groups.
(A) Driving forces involved in peptide-based co-assembly: Aromatic interactions, hydrogen bond, hydrophobic interactions, electrostatic interactions and Van der Waals force. (B) Commonly used amino acids and molecules in peptide-based co-assembly.
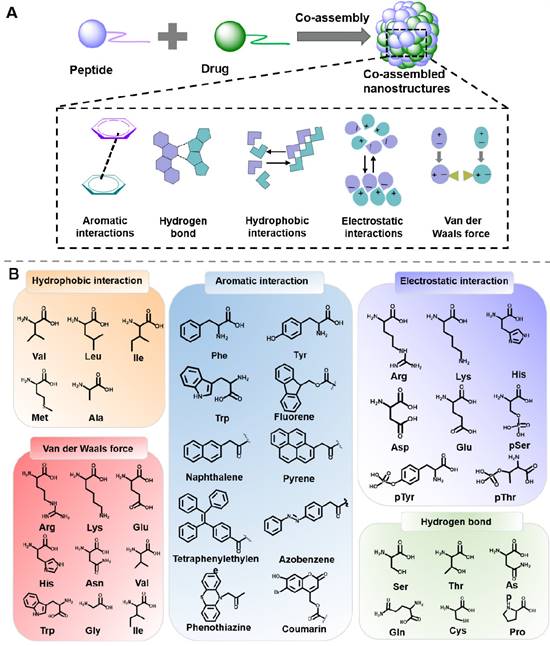
3.3 Electrostatic interactions
Electrostatic interaction is generally not directional but is another important interaction for prompting assembly. Research suggested that the shielded Coulomb effect between positive and negative charges would trigger the formation of ion pairs. However, the electrostatic interaction is always influenced by environmental conditions, which will be weakened by hydrated ions and the charge shielding effect from salt [82]. In the assembly process of charged peptides, the electrostatic interaction plays an unsubstitutable role for regulating the assembly behavior. In 1993, Rich and his colleagues discovered the simple self-complementary EAK16 peptide, which consisted of oppositely charged amino acids. EAK16 could spontaneously assemble to form a fairly stable macroscopic membrane [83]. Electrostatic interaction also plays an indispensable role in the co-assembly of peptides and peptides, or peptides and drugs. For example, Webber et al. designed tetrapeptides (KWKW, DWDW) with opposite charge. The electrostatic interactions between DWDW and KWKW induced the hierarchical structures with favorable mechanical properties [84]. Zhong et al. designed a series of novel peptide derivatives 1-RGDHn (Nap-GFFYGRGDHn), which could co-assemble with doxorubicin (DOX) to confers 1-RGDHn hydrogels under electrostatic interactions [85]. In a similar way, charged peptides could be utilized to design various carriers for drug delivery with complementary charges in the future.
3.4 Hydrogen bond
Hydrogen-bond is considered crucial for stabilizing the structural organization of protein and peptide. Peptide possesses hydrogen bond formation sites, such as carboxyl, and amide groups in the side chains and backbone of the peptides. When there are strong hydrogen bonds in the system, peptide tends to form β-sheet structures. However, the strength of the hydrogen bond can be significantly influenced by the direction and distance between hydrogen acceptor and donor pairs. Diphenylalanine prefers to form nanotubes in an aqueous environment while long nanofiber in nonpolar toluene can be ascribed to the different hydrogen bonding and polarity in various solvents [86]. For example, Yan et al. validated that solvents capable of forming hydrogen bond, including ethanol, DMF, and acetone, can expedite the nanofiber formation of diphenylalanine [87]. Their groups also summarized that the cyclic dipeptides possessed excellent hydrogen bonding-forming ability, which exhibited great value in drug delivery and cancer therapy. Besides, the hydrogen-bond between hydrogen atoms and electronegative atoms in peptides and drugs could stabilize the formed nanostructures. For instance, by integrating cyanuric acid and peptide, Zhang et al. designed a new amphiphilic peptide (CA-C11-GGGRGDS). Once mixing this peptide with methotrexate (MTX), the complementary hydrogen bonding interaction between cyanuric acid in peptide and MTX would facilitate the increase of hydrophobicity of the system, thus leading to peptide/MTX nanostructures [88]. Yang et al. employed the co-assembled strategy to fabricated doxorubicin-loaded gel, both the hydrogen bond and aromatic π-π stacking played the critical role in the co-assembly process [89].
3.5 Van der Waals force
Van der Waals force belongs to a short-range force without directivity and selectivity. Since the strength of van der Waals forces is weaker than that of hydrogen bonds, it rarely acts as the main force to promote peptide self-assembly, but always cooperates with other interactions as a driver. Wang and colleages utilized steered molecular dynamics (MD) simulations and umbrella sampling techniques to investigate the formation mechanism of KIIIIK in their study [90]. The findings revealed that the formation of the intra-sheet structure was influenced by both van der Waals and electrostatic interactions, whereas the inter-sheet structure was mainly governed by the van der Waals force. Van der Waals forces are also a crucial force to regulate the co-assembly process between peptides and drugs. Li et al. designed a hybrid supramolecular hydrogel (Lev/Dex-SA-RGD) through the co-assembly of dexamethasone-peptide amphiphile (Dex-SA-RGD) and antibiotic levofloxacin (Lev) [91]. Further study indicated that the balance of electrostatic forces and Van der Waals forces play an important role in the process of co-assembly.
In general, these weak interactions were always prone to be influenced by the surrounding environment, such as pH, temperature, solvent, and stimuli. In the co-assembly process, it is always necessary to rely on the cooperation of multiple interactions to produce multifunctional and stable nanostructures. The research group represented by Yan et al. has constructed a variety of structurally stable supramolecular delivery systems through the cooperation of a variety of non-covalent forces between peptides and drugs [92]. Overall, the non-covalent forces play an indispensable role in the co-assembly of peptides and drugs, which would be significantly considered when designing co-assembled nanostructures for drug delivery.
4. Co-assembly of peptide and drug molecules for cancer therapy
In the early stage of cancer, surgical treatment is the main treatment for low-risk patients. However, in the middle and late stages, systematic treatment is of significant importance. Systematic treatment of cancer includes chemotherapy, phototherapy, gene therapy, radiotherapy, immunotherapy, and combination therapy. In order to achieve the optimum therapeutic effects, carrier-based delivery is usually needed. Thanks to the good biocompatibility, tunable structures and excellent biodegradability of peptides, the peptide assemblies-based delivery system exhibits broad application prospects in drug delivery.
Great progress has been made in the delivery of anticancer molecules with peptide assembly strategies, which is attributed to good biocompatibility, enhanced accumulation in tumor sites, and cell permeability. Traditional encapsulation is always troubled by drug leakage, affecting efficacy. Encouragingly, the co-assembly delivery system formed by the interaction between peptide and drug molecules has been approved to conquer the above problems. The co-assembly strategy exhibits the following advantages: (Ⅰ) Enhanced stability of therapeutic agents in vivo; (Ⅱ) Controlled release profile of therapeutic agents; (Ⅲ) Improved drug loading efficiency; (Ⅳ) Enhanced targeting capability; (Ⅴ) Excellent therapeutic efficacy. The following focused on the recent research about the co-assembly of peptides with various drug molecules (Table 1).
List of classification of co-assembled peptides and therapeutic agents for cancer therapy.
| Classification of co-assembly | Peptide moieties | Active moieties | Factors triggering co-assembly | Cell lines | Ref |
|---|---|---|---|---|---|
| Chemotherapeutic agents-based co-assembly | ATKTA-S-S-ATKTA | Curcumin | Hydrogen bond | HeLa | 98 |
| Nap-GFFYGRGDH | DOX | Electrostatic interaction | A549 | 85 | |
| Ac-ATK(C18)DATGPAK(C18)TA | DOX | Hydrophobic interaction | PC-3 | 99 | |
| Fmoc-FK (FK)/ Fmoc-FKK (FKK) | DOX | Aromatic interaction | MDA‐231 | 100 | |
| CA-C11-GGGRGDS | MTX | Hydrogen bond | HeLa | 88 | |
| IDM-GFFYGRGDH | IMD+DOX | Electrostatic interaction | A549 | 101 | |
| FKFEY-YSV | HCPT+ YSV | π-π stacking and electrostatic interactions | A549 | 102 | |
| Nap-FFYERGD | Cisplatin+ Irinotecan | Coordination interaction | A549 | 103 | |
| HCPT-FFERGD | HCPT+ Cisplatin | Coordination interaction | A549 | 104 | |
| Rh-GFFYERGD | Rhe+ Cisplatin | Coordinated and hydrophobic interactions | A549 | 105 | |
| Radiosensitizer-based co-assembly | Npx-DFDFDEDY | Cisplatin | Electrostatic interaction | A549 | 114 |
| Ce6-R9-125I-RGD | 125I+Ce6+ miR-139-5p | Electrostatic interaction | HeLa | 115 | |
| NIA-D1 | NIA+ R848 | Non-covalent interaction | MC38 | 117 | |
| FFRGD | H2S+IR | π-π stacking | H1299 | 118 | |
| Gene drugs-based co-assembly | Retro-Inverso CADY-K | siRNA | Hydrophobic interaction | U87 | 123 |
| KALA-2LMWP-NLS | pDNA | Electrostatic interaction | MCF-7 | 124 | |
| TR4 | pDNA | Non-covalent interactions | HeLa | 125 | |
| Fmoc-RRMEHRMEW | siRNA | Electrostatic interaction | HeLa | 126 | |
| Fmoc-RRMEHRMEW | AS1411 | Non-covalent interaction | T47D | 127 | |
| GRVEVLYRGSW GRVRVLYRGSW | siRNA | Electrostatic interaction | MCF-7 | 128 | |
| STR-H16R8 | siRNA | Electrostatic interaction | HCT 116 | 129 | |
| STR-HK | siRNA | Electrostatic interaction | A549 | 130 | |
| TNCP | ASN | Electrostatic interaction | MDA-MB-231 | 131 | |
| FC-PyTPA | Bcl-2 siRNA+PyTPA | Electrostatic interaction | HeLa | 132 | |
| Chol-HHHHHHH-AKRGARSTA | siRNA+1MT | Non-covalent interaction- | 4T1 | 133 | |
| Phototherapeutic agents-based co-assembly | NapFFKYp | ICG | Coordinated intermolecular interaction | HeLa | 136 |
| Z-Histidine-Obzl | Biliverdin | Hydrophobic, π-π and hydrogen bonding interaction | MCF7 | 137 | |
| Fmoc-L3-OMe | m-THPP | π-π stacking and hydrophobic interaction | MCF-7 | 140 | |
| H-FF-NH2·HCl, Fmoc-K | Chlorine6 | Electrostatic, Hydrophobic, and π-π interaction | MCF7 | 92 | |
| Fmoc-L-L | Chlorine6 | Coordination, hydrophobic and π-π stacking interaction | MCF7 | 141 | |
| Fmoc-L3-Arg | THPP | Hydrophobic, electrostatic, hydrogen bond and electrostatic interactions | 4T1 | 144 | |
| TAT-IR780 | IR780+Dox | π-π stacking and hydrophobic interactions | 4T1 | 145 | |
| RKDVY(TP5) | TP5+ ICG | Non-covalent interaction | Pan02 | 146 | |
| LND-K | TPPS4+LND | Electrostatic interaction | A375 | 147 | |
| Immunotherapeutic agents-based co-assembly | 10 K-Adpgk | Adpgk | Electrostatic interaction | MC-38 | 154 |
| Epitope-R8 | Epitope | Non-covalent interaction | B16-OVA | 155 | |
| AC-KLVFFAL-NH2 | c-di-GMP | Electrostatic interaction | B16-F10 | 156 | |
| Fbp-GDFDFDYD(E, S, or K)-ss-ERGD | OVA | Non-covalent interaction | E.G7-OVA | 157 | |
| Fbp-GDFDFDYDK(γE)2-NH2 | OVA | Hydrogen bond and π-π interactions | E.G7-OVA | 158 | |
| HmA | Antibodies | Coordinative interaction | HeLa | 159 | |
| ECPs | K-OVA257-264+E-OVA323-336 | Non-covalent interaction | E.G7-OVA | 161 | |
| DEAP-DPPA-1 | DPPA +NLG919 | Non-covalent interaction | B16-F10 | 162 | |
| PCP | R848+DOX | Hydrophobic-hydrophilic interaction | 4T1 luciferase | 163 | |
| AmpF, AmpFY, AmpFC919 | NLG919+125I | Non-covalent interaction | 4 T1 | 164 |
4.1 Co-assembly of chemotherapeutic agents and peptide
4.1.1. Chemotherapeutic agents-peptide co-assembly for chemotherapy
Chemotherapy lays a foundation for traditional cancer treatment. Chemotherapeutic drugs exert their anti-cancer efficacy through destroying the cell cycle process. According to the mechanism of curative effects, chemotherapeutic drugs can be classified into five categories, including alkylating agent, antimetabolic agent, tubulin-binding drug, topoisomerase inhibitor, and antineoplastic antibiotics [93-97]. Alkylating agent includes nitrogen mustards, platinum drugs, and oxazsaphosphorines, which always destroy cancer cells by covalently binding with the DNA and RNA of cancer cells. With a similar structure to metabolites in vivo, antimetabolic agents exhibit their efficacy by specific antagonism with the essential metabolite. Pyrimidine drugs, purine drugs, and antifolates are commonly used as antimetabolic drugs. Taxanes and vinca alkaloids belong to tubulin-binding drugs, which can inhibit the formation of the microtubule by binding to tubulin and further kill cancer cells. Topoisomerase inhibitors can significantly inhibit topoisomerase activity and promote the breakage of DNA strand breakage. Antineoplastic antibiotics kill cancer cells by inserting into DNA at particular sites to further destroy DNA strands. However, there are still some major limitations in the application of chemotherapeutic drugs. Drug resistance is a major contributor to the failure of chemotherapy, wherein drug efflux, gene mutations, enhanced DNA repair capability, and increased anti-apoptotic capability are often attributed as causes. Besides, chemotherapy also associates with serious side effects, poor targeting ability, and limited therapeutical efficacy.
However, the co-assembly of peptides in chemotherapeutic agents displays promising potential for addressing the issues of poor targeting and toxic side effects encountered with chemotherapy. The co-assembly strategy can exert a significant influence on the physicochemical properties and targeting ability of the drug delivery system. On the one hand, the co-assembly of peptides and chemotherapeutic agents works to tune the micromorphology and the mechanical properties of the delivery system; on the other hand, this co-assembly strategy can enhance the targeting ability and anti-tumor efficacy of chemotherapeutic agents.
Firstly, the micromorphology of nanostructures can be tuned by utilizing the co-assembled strategy, which will meet the different requirements of the delivery system. For example, He and his colleagues designed a cystitis-bridged peptide (ATKTA-S-S-ATKTA, abbreviated CBP), which could co-assemble with hydrophobic antitumor drug curcumin (CCM) to form glutathione (GSH)-responsive CCM-loaded micelles (CCM-CBP). The aromatic groups of curcumin interacted with the hydrophobic moiety of the peptide and formed new intermolecular hydrogen bonds, thus leading to microscopic morphological transformation from fiber to micelle. Additional findings revealed that CCM-CBP micelles displayed improved cellular uptake by cells and effectiveness against tumors [98]. Zhong et al. designed Naphthylacetic acid-conjugated peptides (Nap-1), which consisted of self-assembled sequences, RGD residues, and histidine residues. Nap-1 could co-assemble with DOX·HCl through electrostatic interactions to form nanofiber for controllable drug release and efficient inhibition of cancer cells [85]. Zhao et al. described CAP-NPs, which were formed by the co-assembly of a FAP-a responsive amphiphilic peptide (Ac-ATK(C18)DATGPAK(C18)TA, CAP), and Dox. After the co-incubation of CAP with Dox, the fiber structure was gradually transformed into stable nanospheres under the hydrophobic driving force, displaying the indispensable role of the hydrophobic drug in the process of co-assembly [99].
Secondly, the co-assembly strategy helps drugs target tumor cells or subcellular organelles and prolongs drug residence time at tumor sites, thus leading to enhanced therapeutical efficacy. Yang and his colleagues prepared Fmoc-FK (FK) and Fmoc-FKK (FKK), which could co-assemble with Dox in different ratios to obtain two kinds of peptide-Dox nanoparticles. These nanoparticles demonstrated excellent uptake of tumor cells due to the uniform particle size distribution (50-100 nm) and positive charge in the nanoparticles [100]. Significantly, introducing targeting groups into the peptide structure for tumor overexpression receptors can enhance the targeting performance of the drug delivery system. Zhang et al. designed a novel amphiphilic cyanuric acid-peptide conjugate, which could form nanorods or nanofibers with different concentrations of methotrexate (MTX) under the interaction of hydrogen bonds between cyanuric acid in peptide and MTX. This MTX-loaded nanostructure showed superior anti-tumor effects compared with MTX, and the effects could be ascribed to the excellent targeting ability of the amphiphilic peptide [88].
4.1.2. Chemotherapeutic agents-peptide co-assembly for combined chemotherapy
Combination chemotherapy depends on different chemotherapeutic drugs at a time to treat cancer, and by affecting the cell repair of cancer cells or different stages of the reproductive cycle, the efficacy of chemotherapy can be enhanced. The application of combination chemotherapy is guided by several general principles: 1) The drugs should be dosed appropriately to avoid superimposed toxicity; 2) They should exhibit different mechanisms and minimal cross-resistance; 3) Synergistic therapeutic effects should be observed at optimal ratios of drug doses; and 4) The drugs should have similar solubility to ensure efficient delivery to cells concurrently.
Peptide-based co-assembly offers a promising strategy to integrate multiple chemotherapeutic agents into a single nanosystem, enhancing the therapeutic effects and reducing adverse effects. Zhong et al. aimed to maximize the effectiveness of chemotherapy, designed an indomethacin (IDM)-peptide conjugate (IDM-GFFYGRGDH, denoted as IDM-1) for boosting the therapeutic efficacy of chemotherapeutics Dox. IDM-1 and Dox were co-assembled to produce IDM-1/DOX hydrogels, which had lower minimum gelation concentrations (MGCs) (1.5 wt%) than IDM-1 (2.0 wt%). The decrease of MGCs of IDM-1/DOX hydrogels indicated the Dox played a bigger part in assembly process. Further results showed that IDM-1/DOX hydrogels could continuously release Dox to synergistically inhibit A549 cancer cells [101]. Recent research suggested that tyroservatide (YSV) exhibited antitumor efficacies despite the disadvantage of large dosages. In the context of the study, their group described a YSV moiety-containing octapeptides (FKFEYYSV, abbreviated as 1-YSV). Intriguingly, 1-YSV demonstrated a stable co-assembly with hydroxycamptothecin (HCPT), which could confer 1-YSV/HCPT hydrogel with excellent mechanical properties and enhanced therapeutic efficacy [102].
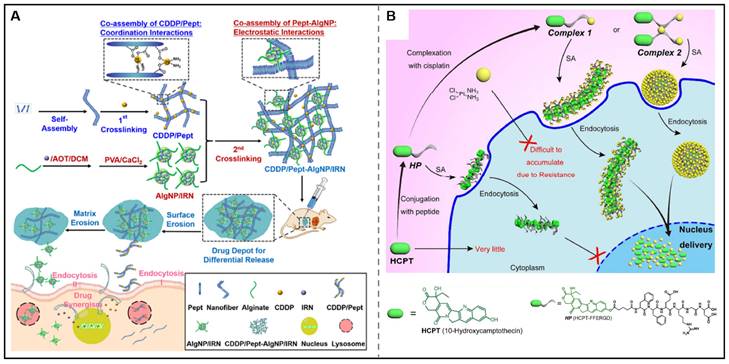
Focusing on the temporal control in combined chemotherapy, the Zhong group also showed a nanocomposite hydrogel for the differential release of two chemotherapeutic drugs. In this design, a hydrogelator (Pept) was assembled with cisplatin via coordination interactions to form CDDP/Pept matrix. Additionally, emulsion cross-link technique was utilized to prepare nanoparticles loaded with irinotecan (IRN). Through a co-assembling process, CDDP/Pept-AlgNP/IRN nanocomposite hydrogel was obtained through the electrostatic interactions between CDDP/Pept and AlgNP/IRN. Notably, this nanocomposite hydrogel could be served as a platform for the tunable release of CDDP and IRN by tailoring the fraction ratios of AlgNP/IRN in the hydrogel, showing superior synergistic antitumor effects in vitro and in vivo (Figure 5A) [103]. However, only delivering the drugs into the tumor cells couldn't yield optimal therapeutic effects as the action sites of some drugs were in the intracellular subcellular organelles. Yang's research group has made great progress in the utilization of co-assembly to improve the subcellular targeting ability of chemotherapeutic agents. They designed and synthesized HCPT-peptide conjugate (HCPT-FFERGD, abbreviated as HP). HP could interact with different equivalents of cisplatin and self-assemble into distinct nanostructures with different micromorphology (Figure 5B). The assembled nanostructure could be served as a “Trojan horse” carrier that could deliver dual anticancer drugs to reach the nucleus to efficiently kill tumor cells [104]. After that, their research group employed the strategy of co-assembly for the nuclear delivery of rhein and cisplatin. They synthesized rhein-peptide conjugate (Rh-GFFYERGD and Rh-GFFYERGE), which could co-assemble with cisplatin to form Rh-gel. In vitro drug release experiments indicated that the cumulative release of cisplatinum was 34.9, 31.2, and 23.9% within 16 h from Rh-gel formed by incorporation of 4, 6, and 8 eq. cisplatinum, respectively. Rh-gel not only exhibited excellent nuclear accumulation properties but also displayed superior anti-tumor efficacy in vitro and in vivo [105]. We envision co-assembly strategy can be further used in the clinic to improve the therapeutic effects of chemotherapeutic agents for combined chemotherapy.
Even though the stability and targeting ability of chemotherapeutic drugs have been greatly improved through co-assembly strategy, the effectiveness of chemotherapy is still unsatisfactory for some cancers. According to specific tumor types to design tumor microenvironment responsive co-assembled peptide delivery system may be an effective strategy to enhance curative effects and reduce side effects of chemotherapeutic drugs.
4.2 Co-assembly of radiosensitizer and peptide
4.2.1. Radiosensitizer-peptide co-assembly for radiotherapy
Radiotherapy is one of the main methods to treat cancer, and its cure rate is about 50%. For patients with laryngocarcinoma, non-small cell lung cancer, gliomas or sarcomas, radiotherapy exhibits significant therapeutic effects and the survival rate is higher than that of other therapies. However, radiotherapy has been found to have unsatisfactory results in treating sarcoma, gastric cancer, and other types of cancer. Ionizing radiation, which is produced by X-ray, electron, proton, or other particle beam is greatly related to the effectiveness of radiotherapy. Reactive oxygen species produced by ionizing radiation can destroy DNA strands and inhibit the cell cycle, leading to tumor cell death [106, 107]. Besides, endogenous danger signals can promote the release of cytokines and further boost the presentation of tumor-associated antigens, inducing the diversity of host T cells. Although the superiority of these novel radiotherapy strategies in tumor therapy, they can still cause various side effects, such as the occurrence of secondary tumors, radiation-induced pneumonia, lymphedema, and spinal cord injury [108-110].
Chemotherapeutic agents-peptide co-assembly for combined chemotherapy. (A) Schematic illustration of the co-assembly of CDDP/Pept-AlgNP/IRN nanocomposite hydrogel loaded with dual drug cisplatin (CDDP) and irinotecan (IRN) for combination therapy. Adapted with permission from [103], copyright 2020, Elsevier Ltd. (B) Schematic illustration of supramolecular nanostructures for nuclear delivery of 10-hydroxycamptothecine (HCPT) and cisplatin (CDDP) against cancer cells. Adapted with permission from [104], copyright 2017, American Chemical Society.
Recently, nanomaterials have gained favorable achievements in enhancing radiotherapy sensitivity and reducing side effects. Among them, peptide assemblies have gained encouraging results in sensitive radiotherapy. For example, Ding et al. designed a curcumin-peptide conjugate, which could self-assemble to form Cur-SNF nanofiber. The nanofiber showed a superb performance in amplifying the radiosensitivity of cancer cells to ionizing radiation [111]. Liu and other groups also reported impressive results of peptide assemblies in sensitizing radiotherapy [112, 113]. The peptide-based co-assembly approach also provided an available strategy for improving the radiotherapy effects. Liu and coworkers developed a novel supramolecular hydrogel combining naproxen (Npx)-peptide conjugate (Npx-FFEY) and radiosensitizer cisplatin. The co-assembled supramolecular hydrogel exhibited enhanced radiosensitization effects by promoting the formation of Pt-DNA adducts and inhibiting the activity of COX-2 [114].
4.2.2. Radiosensitizer-peptide co-assembly for combined radiotherapy
Hypoxic tumor cells are prone to radiotherapy resistance. Radiotherapy combined with other therapies may induce promising outcomes. Recent research indicated that radiotherapy combined with PDT exhibits encouraging results, such as improving the quality of life of patients, reducing side effects, and enhancing the sensitivity of radiotherapy. 125I radioactive particles display huge advantages in radiotherapy due to their long duration time in tumors and negligible toxicity to normal tissue. Accordingly, Dai et al prepared an R9-based peptide derivative that integrated 125I-labeled cRGD and Ce6 to further co-assemble with miR-139-5p to afford nanoparticles (Ce6-R9-125I-RGD-MNPs) [115]. The nanoparticles exhibited excellent targeted ability towards tumor cells and enhanced combined therapeutical efficacy between photodynamic therapy and radiotherapy.
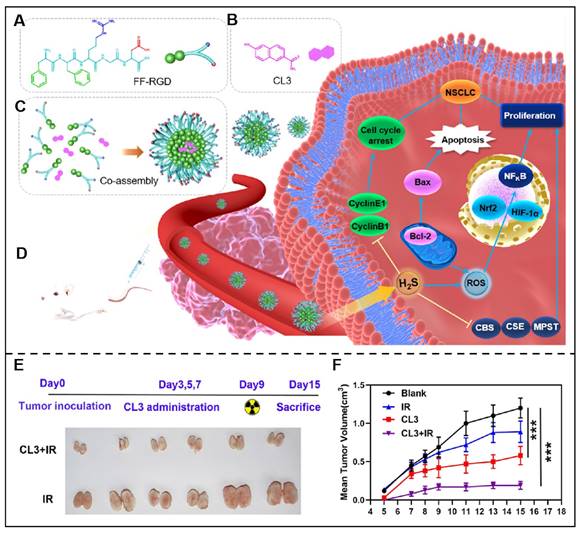
Radiotherapy combined with immunotherapy integrated efficient radiotherapy effects and immunological effects, displaying distinctive superiorities for tumor therapy. For example, Schoenfeld and his colleagues studied the efficacy of ipilimumab and 65 courses of radiation in 47 patients with consecutive metastatic melanoma and the results indicated a promising synergistic efficacy of this therapeutic regime [116]. Inspired by these research findings, Gao et al. designed a novel peptide derivative NIA-D1, which integrated radio-sensitizer 2-(2-nitroimidazol-1-yl) acetic acid (NIA), matrix metalloproteinase-2 (MMP-2) responsive peptide sequence and PD-L1 antagonist. This peptide derivative co-assembled with dendritic cells (DCs) activators toll-like receptor (TLR) 7/8 ligand R848 and further formed NIA-D1@R848 nanoparticles. Once NIA-D1@R848 reached tumor sites, it would be decomposed under the overexpressed MMP-2 in the tumor microenvironment, and NIA-PLG, PD-L1 antagonist, and R848 were then released. The released NIA-PLG would promote the released antigen under radiation, and R848 boost the maturation of DC, while the PD-L1 antagonist would further alleviate T cell suppression, and they work together to induce cancer cell death [117]. Tian et al. also constructed a novel co-assembled delivery system by integrating FFRGD and H2S donors (CL2/3) for effcienct combined chemo-radiotherapy (Figure 6) [118].
Peptide-based co-assembly for sensitized radiotherapy. (A) Chemical structure of FFRGD peptide. (B) Chemical structure of H2S Donor (CL3). (C) Schematic illustration of FFRGD peptide co-assembled with H2S into nanocarriers. (D) Schematic illustration of the co-assembled nanocarriers for cancer therapy. (E) Schematic illustration of representative xenografts were extracted from 2-Gy radiation (IR) or co-assembled nanocarriers+IR treated groups of nude mice. (F) Time curve of average tumor volume after nude mice were received with 2-Gy radiation (IR) or co-assembled nanocarriers+IR. Adapted with permission from [118], copyright 2022, American Chemical Society.
The integration of radiotherapy with co-assembly strategy have broken the limitations of radiotherapy, such as the reduced toxicity, enhanced therapeutical efficacy and extend the application range in combination. The efficacy of radiotherapy is heavily reliant on the quality of imaging technology employed. The combination of peptide-based co-assembly with advanced imaging technology may bring significant development for cancer treatment in further.
4.3 Co-assembly of gene therapeutic agents and peptide
4.3.1. Gene therapeutic agents-peptide co-assembly for gene therapy
Gene therapy based on small interfering RNA (siRNA), plasmid DNA (pDNA) and RNA/DNA aptamers have become a burgeoning method for tumor therapy [119, 120]. Among them, gene therapy represented by siRNA-based therapy has been widely studied thanks to the significant advantages of anti-tumor therapy. Although siRNA has limited protein targets, it can interfere with almost all gene transcripts. According to phase I clinical trials, siRNA can prevent various gene targets and lead to cancer cell death, which has been verified in patients with cancer [121]. siRNA and other gene therapeutic agents are prone to degradation by enzymes within the body and exhibit instability in blood circulation. In addition, due to their larger size and negative electricity, it is difficult for gene therapeutic agents to cross cell membranes freely. Thus, it is necessary to find a suitable method to improve the in vivo stability and delivery efficiency of gene therapeutic agents.
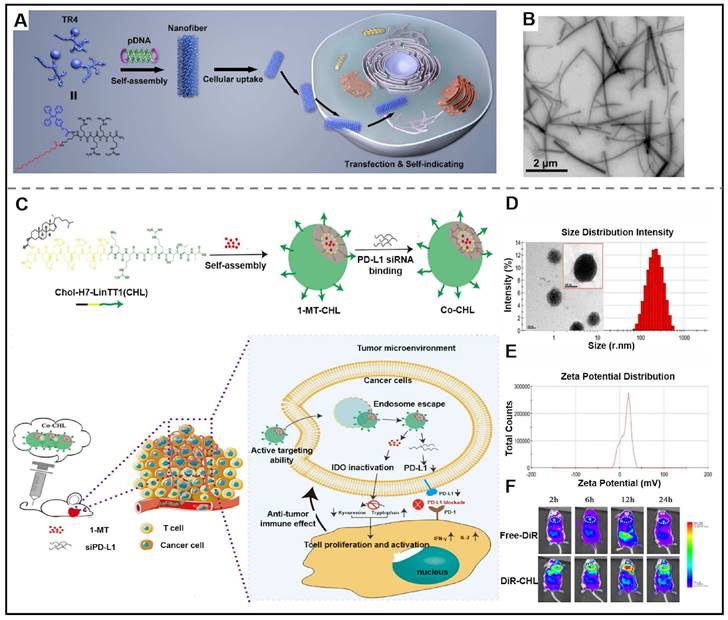
Recently, researchers have designed new delivery vectors to improve its shortcomings, bringing the utilization of gene therapeutic agents in clinical cancer treatment into reality [122]. Supramolecular peptides are increasingly recognized as the ultimate delivery carrier due to their superior capacity to enhance the stability of gene therapeutic agents and promote their delivery efficiency, amongst all other delivery carriers. Peptides can be co-assembled with gene therapeutic agents to form nanostructures with diverse structures, such as micelles, nanoparticles, nanotubes, and nanofibers. The driving forces of co-assembly between peptides and gene therapeutic agents mainly include non-covalent interactions, such as hydrophobic interaction, hydrogen bonds, electrostatic interaction, and so on. It is noteworthy that the peptide used for efficiently delivering gene therapeutic agents needs meet specific structural requirements, which include (Ⅰ) a hydrophobic sequence for endowing peptide with self-assembling; (Ⅱ) a hydrophilic segment for balancing the amphiphilicity; (Ⅲ) a positively charged amino acid residues for expeditiously binding with siRNA and improving the loading stability of siRNA; (Ⅳ) targeted groups for improving targeting ability towards cancer cells. The utilization of co-assembly between peptide and gene therapeutic agents is a breakthrough in improving the in vivo stability of therapeutic agents. For instance, Deshayes et al. described a novel D-cell penetrating peptide (RICK), which was the retro-inverse transformation of L-parental CADY-K. RICK/siRNA assemblies were able to prevent the degradation of siRNA and inhibit gene expression [123]. Ramezani et al. presented a nanoplatform consisting of peptides functionalized with aptamers. Firstly, an arginine-rich peptide (LWMP) was functionalized with KALA and a nuclear localization signal (NLS) to obtain a novel peptide KLN (KALA-2LWMP-NLS). And then, the KLN peptide was assembled with plasmid DNA (pDNA), and further modified with AS1411 aptamer (Apt) to form KLN/pDNA/aptamer nanoparticles. The results indicated that the developed nanoplatform exhibited excellent stability which could prevent pDNA from degradation. The nanoparticles also showed satisfactory targeting ability towards specific tumor cells and enhanced cellular uptake, thus demonstrating superior gene transfection [124]. Adopting a similar strategy, Liang et al. synthesized a tetraphenylethene (TPE)-conjugated peptide TR4, which co-assembled with plasmid DNA (pDNA) into pDNA@TR4 nanofibers (Figure 7A-B). pDNA@TR4 nanofibers were stabilized by the noncovalent interactions between pDNA and TR4, thus leading to enhanced stability of gene/vector complexes [125].
In addition, the co-assembly of peptides and gene therapeutic agents can improve the delivery efficiency, boost gene transfection efficiency and improve therapeutic efficacy. For example, Li et al. described peptide Wpc, which was obtained by modifying the Met residues in Fmoc-RRMEHRMEW with reducible cross-linkers. Peptide Wpc could co-assemble with siRNA at the optimum peptide/siRNA ratio to form peptide-siRNA nanoparticles. These nanoparticles demonstrated improved cellular uptake of siRNA and elevated transfection effectiveness. The in vivo experimental results showed that peptide-siRNA nanoparticles could significantly inhibit tumor growth with low side effects [126]. In addition, their group also designed a novel co-assembled Wpc/AS1411 nanocarrier for efficient delivery of aptamer AS1411 to enhance anticancer therapeutical efficacy [127]. Among multifunctional peptides, cell-penetrating peptides, such as arginine-containing peptides, displayed great potential in siRNA delivery due to their excellent transmembrane ability. Mollenhauer and co-works construed a siRNA nanocomplex delivery system based on the respective co-assembly of two DMBT1-derived-peptides with siRNA. They synthesized a DMBT1-derived peptide (SRCRP2-11, GRVEVLYRGSW) to bind with tdTomato1 siRNA. In addition, SRCRP2-11-R was synthesized by replacing the glutamic acid (E) at position 4 of SRCRP2-11 with an arginine. By changing the ratios of peptide and siRNA, the size of two peptide-siRNA complexes could be tuned and the two peptide-siRNA complexes exhibited efficient gene silencing in MCF7-recombinant cells [128]. Inspired by that superior binding affinity with cytomembrane, Chen et al described a novel arginine-rich peptide NP1 (STR-H16R8) to aid siRNA delivery. Their results indicated that NP1 could co-assemble with siRNA into nanoparticles (<200 nm), which displayed improved siRNA knockdown efficiency in 3D spheroids of colon cancer cells [129]. To enhance the cellular uptake of siRNA, their group designed peptide/siRNA complexes by combining STR-HK peptides with siRNA, which apparently enhanced the inhibitory effects on tumor proliferation [130]. Xia et al. designed a multifunctional peptide-conjugated TDNCP, which contains the following parts: (Ⅰ) targeted peptide sequence (DGR or RGD); (Ⅱ) nuclear targeting sequence (KRRRR); (Ⅲ) cell-penetrating peptide (RRRR); (Ⅳ) an AIE molecule. TDNCP co-assembled with DNA oligonucleotide (ASO) into stable TNCP/ASO-NPs through electrostatic interaction. TNCP/ASO-NPs showed a strong inhibition on tumors and a great interference effect thanks to their outstanding targeting function [131].
Gene therapeutic agents-peptide co-assembly for cancer therapy. (A) Schematic illustration of the co-assembled pDNA@TR4 nanofibers by using a short peptide derivative (TR4) and plasmid DNA (pDNA) for traceable gene delivery. (B) TEM image of pDNA@TR4 complexes, bar: 2 μm. Adapted with permission from [125], copyright 2017, American Chemical Society. (C) Schematic illustration of Co-CHL NPs, by the assembly of CHL (Chol-HHHHHHH-AKRGARSTA), siRNA of programmed cell death ligand 1 (siPD-L1) and 1-methyl-DL-tryptophan (1MT) and the mechanism of Co-CHL NPs for cancer treatment. (D) The size distribution characteristic of Co-CHL NPs. (E) Zeta potential of Co-CHL NPs. (F) In vivo images of 4T1 tumor-bearing mice after receiving tail vein injection of free DiR or DiR-loaded Co-CHL NPs in different times. Adapted with permission from [133], copyright 2019, American Chemical Society.
4.3.2. Gene therapeutic agents-peptide co-assembly for combination gene therapy
Current clinical research on single-agent therapy has displayed considerable therapeutical efficacy, but its side effects are nonnegligible. Multiple-agent therapy works to synergistically kill cancer cells via different action mechanisms. In terms of combination therapy in anti-tumor, there are several reports on gene therapeutic agents-based supramolecular peptide co-assembly in combined gene therapy. Xia et al. designed a peptide-conjugated AIEgen (FC-PyTPA), which co-assembled with siRNA into FCsiRNA-PyTPA through electrostatic interactions. In vitro and in vivo experiments demonstrated that the production of ROS, gene interference of CsiRNA and formation of nanofibers synergistically promote cancer cell death [132]. Gao et al. designed a novel drug delivery system (Co-CHL NPs) based on cholesterol-peptide conjugate (Chol-HHHHHHH-AKRGARSTA, CHL) for efficient delivery PD-L1 small interfering RNA (siPD-L1) and 1-methyl-DL-tryptophan (1MT). Co-CHL NPs exhibited synergistic antitumor immune response thanks to the dual blockade of an immune checkpoint of siPD-L1 and 1MT (Figure 7C- F) [133].
Although supramolecular peptide assembly displays huge potential in the delivery of gene therapeutic agents, peptide assembly-mediated gene therapy has not been applied in clinical due to several important reasons. First, the designed co-assembly system should be stable and capable of resisting the degradation of enzymes in blood circulation. To this end, the driver of the co-assembly should not only include electrostatic forces, but also other non-covalent forces, such as hydrophobic interaction and hydrogen bonds. Second, multifunctional peptides should be designed to efficiently target and bind with different tumor cells to increase cellular internalization. Last but not least, the assembly should respond to the intracellular environment and precisely release siRNA or DNA in target sites. The co-assembly for gene therapy has gained some advances, which can be used for tumor therapy and gain satisfactory results as the development of structural simulation and modification and the transport mechanism of the peptide assemblies.
4.4 Co-assembly of phototherapeutic agents and peptide
4.4.1. Photothermal conversion agents-peptide co-assembly for photothermal therapy
Phototherapy mainly includes photothermal therapy (PTT) and photodynamic therapy (PDT), which is a potential therapeutic method to enhance anti-tumor efficacy by utilizing photosensitizers and external laser irradiation. PTT is a new phototherapy method different from PDT in the treatment of tumors, which achieves superior anti-cancer efficiency by employing photothermal conversion agents (PTCAs) that generate vibrational energy (heat) when exposed to NIR light [134, 135]. The heat generated by light could increase local temperature and induce local tumor tissue ablation without affecting normal tissues. Although some progress has been made in phototherapy, its clinical applications are still challenged by many problems, including certain thermal damage to normal skin tissue and limited therapeutic effects in PTT. In addition, the rapid clearance of photosensitizer greatly weakened its anticancer efficacy. In recent years, researchers have committed to looking for corresponding solutions to the drawbacks existing in phototherapy, and the following methods, such as enhancing the targeting ability, utilizing reduced-temperature PTT, using combination therapies, and designing intelligent responsive PTT were applied.
Many nanomaterials have been exploited for efficiently delivering PTCAs. Among them, the co-assembly of peptides and PTCAs demonstrates higher potential. In situ assembly triggered by over-expressed enzymes, GSH, ions, etc in tumor microenvironment has been a promising approach to cancer treatment. Chen and his colleagues investigated the co-assembly of ICG and NapFFKYp triggered by enzymes. In the presence of over-expressed alkaline phosphatase (ALP) in the tumor microenvironment, NapFFKYp could convert into NapFFKY. And then, NapFFKY co-assembled with ICG into nanofibers. Compared with free ICG, ICG nanofibers showed partial fluorescence signal quenching, augmented photothermal and photoacoustic signal intensity, and increased cellular uptake (Figure 8A-B) [136]. In addition to in situ assembly, in vitro assembly is another typical method for co-assembling peptide and photosensitizers. Yan et al. adapted biliverdin (BV), endogenic NIR-absorbing pigments, to construct a photothermal nanomaterial to enhance the efficiency of tumor diagnosis and treatment. They employed BV, histidine-containing peptide (Z-Histidine-Obzl, abbreviated as ZHO), and Mn2+ to fabricate ZHO/BV/Mn2+ nanoparticles (ZBMnNPs). ZHO was selected as an assembly agent that could coordinate with Mn2+ through the imidazole residue and co-assemble with BV under the aromatic-aromatic interaction. The result was impressive that, ZBMnNPs not only demonstrated selectivity towards tumors with efficient photothermal treatment effects but also displayed excellent photoacoustic and magnetic resonance imaging [137].
4.4.2. Photodynamic sensitizers-peptide co-assembly for photodynamic therapy
PDT is an effective anti-cancer method, which exerts anti-cancer efficacy with the production of ROS by utilizing photosensitizer and external laser irradiation. There are two key parts to this process, one is that the photosensitizer can effectively enter the action site; another one is that the photosensitizer is activated by external laser irradiation [138, 139]. The production of ROS in PDT can lead to cell death. Besides, PDT could also block tumor blood vessels and deprive the nutrition of tumor tissue, resulting in cancer cell death. In addition, PDT presents considerable impacts on the immune system, which inhibit tumor cell proliferation through immunostimulation or immunosuppression.
Photothermal conversion agents-peptide co-assembly for photothermal therapy. (A) Phosphatase-instructed in situ co-assembly of NapFFKYp (2) and indocyanine green (ICG, 1) to form nanofibers (5) for photothermal therapy (PTT). (B) Calcein AM/PI staining of HeLa cells after incubating with indocyanine green (ICG)-doped nanofibers with laser irradiation (808 nm, 1 W/cm2, 5 min). Adapted with permission from [136], copyright 2015, American Chemical Society. (C) Schematic illustration of the Fmoc-L-L/Mn2+/Ce6 nanoparticles (FMCNPs) which were formed by co-assembling of peptide derivative Fmoc-L-L, MRI contrast agent Mn2+ and photosensitizer Ce6 via the cooperation of multiple non-covalent interactions for MRI-guided PDT. (D) Time curve of average tumor volume of MCF7-tumor-bearing mice after receiving various groups treatments. Adapted with permission from [141], copyright 2018, American Chemical Society. (E) Schematic illustration of the supramolecular nanofibers, assembled by LND-peptide conjugate (LND-K) and photosensitizer TPPS4 for the efficient delivery of lonidamine (LND) and synergistic photodynamic therapy. (F) CLSM images of LND-K/TPPS4-treated cells with JC-10 for indicating mitochondrial membrane potential. Red, JC-10 aggregates; Green, JC-10 monomer. Adapted with permission from [147], copyright 2023, Elsevier Ltd.
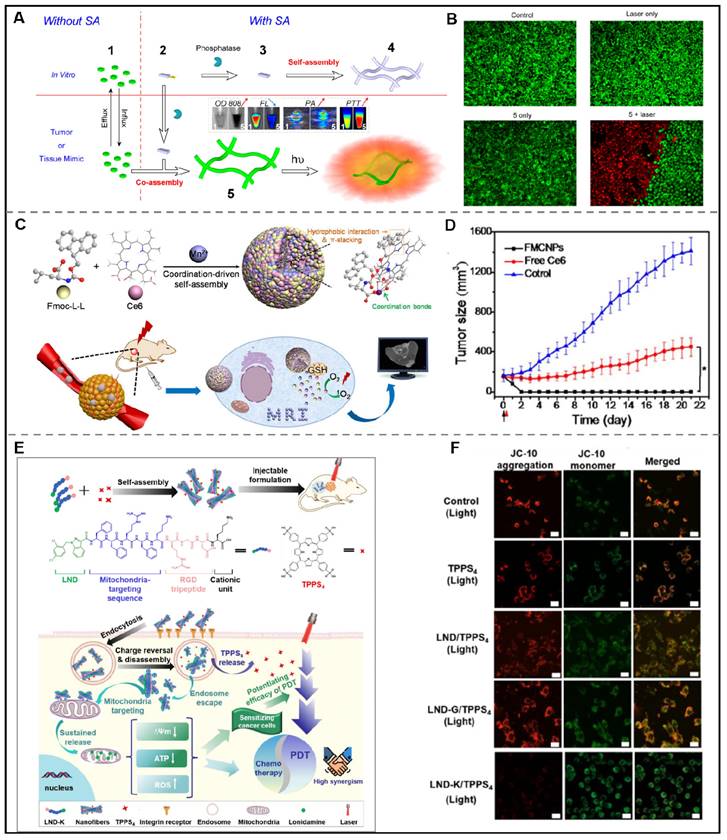
However, the hydrophobicity and non-selective distribution of photosensitizers lead to their aggregation and limited therapeutic effects, which hinders their clinical usage. The peptide-mediated assembly provides a new strategy to solve these problems. Notably, the benzene rings and hydrophobic residues of photosensitive molecules enable it to form a non-covalent force with peptide molecules and further promote the assembly process of peptides. In this context, Bai et al. employed a one-step co-assembly method to construct peptide-based nanoparticles for two-photon PDT. Here, the authors designed a peptide derivative Fmoc-L3-OMe which could co-assemble with m-THPP to form porphine doped peptide-based nanoparticles under the synergistic effect of aromatics and hydrophobicity interactions. Further results revealed that the nanoparticles could exert high 1O2 productivity and tumor cell inhibition effects in vitro under two-photon irradiation [140].
In order to exert optimum PDT efficacy, it is essential for photosensitizers to play their roles to reach tumor sites via passive or active targeting. However, the photosensitizers may be degraded in the systemic circulation due to the complex components in the body fluid, which will hinder the application of nanodrugs for PDT. Metal ion-mediated coordinated assembly may be an effective way to tackle this problem due to the stabilization of nanodrugs and their important role in biological regulation. Recently, Yan et al. developed two peptide derivatives, diphenylalanine (H-F-F-NH2·HCl, CDP) and Fmoc-L-Lys, respectively. When mixed chlorine6 (Ce6) with CDP or Fmoc-L-Lys, uniform spherical CDP/Ce6 nanoparticles or Fmoc-L-Lys/Ce6 nanoparticles would form. The structural stability of the obtained nanoparticles relied on the hydrophobic and π-π interactions between peptide and Ce6. Remarkably, the nanoparticles showed satisfactory PDT efficacy in vitro and in vivo [92]. A similar effect can be seen in the co-assembly of amphiphilic peptide (9-fluorenylmethyloxycarbonyl-L-Leucine, Fmoc-L-L), MRI contrast agent (Mn2+), and photosensitizer (Ce6). Yan and his colleagues created Fmoc-L-L/Mn2+/Ce6 nanoparticles (FMCNPs) by co-assembling Fmoc-L-L, Mn2+, and Ce6. These nanoparticles are more stable due to the involvement of π-π, coordination, and hydrophobic interactions. Under the experimental conditions, FMCNPs exhibited significant tumor eradication in mice. In addition, the anti-tumor efficacy could be monitored by utilizing MRI in the presence of Mn2+ (Figure 8C-D) [141]. Employing the similar strategy, their group described a novel nanodrug integrating photosensitizer, Zn2+, and metal-binding peptide to improve the antitumor PDT [142].
Recent advances demonstrated that tumor hypoxia was one of the primary factors hindering the efficacy of PDT [143]. To tackle this problem, Sun et al. designed an arginine-conjugated peptide derivative 9-fluorenylmethyloxycarbonyl-Leu-Leu-Leu-Arg-OH (Fmoc-L3-Arg). The Arg segment in Fmoc-L3-Arg could be oxidized to NO and further combat the tumor hypoxia. In addition, Fmoc-L3-Arg could co-assemble with 5,10,15,20-Tetrakis (4-hydroxyphenyl) porphyrin (THPP) to form spherical THPP/Fomc-L3-Arg nanoparticles, which could not only rapidly release THPP in the tumor microenvironment and produce ROS, but also produce NO and inhibit mitochondrial function, thus leading to enhanced PDT efficacy [144].
4.4.3. Phototherapeutic agents-peptide co-assembly for combined phototherapy
Phototherapy combined with other treatments may reduce the dosage of therapeutic agents, reverse drug resistance and produce synergistic antitumor effects, which always depned on the co-administration of photosensitizers and other therapeutic drugs. Photosensitizers can induce the generation of heat or ROS under NIR light and then induce the destruction of the tumor, exhibiting great potential in the application of phototherapy-combination therapy. Wang et al. have verified that photosensitizers IR780-instructed tumor therapy could be improved by the chemotherapeutic drug doxorubicin (DOX). They constructed a nucleus-targeting nanoparticle system through the assembly of YGRKKRRQRRRC-IR780 (TAT-IR780) and Dox for synergistic phototherapy and chemotherapy [145]. Besides, phototherapy combined with immunotherapy demonstrates an potential solution to prevent tumor cell metastasis and recurrence, which can be ascribed to the amplified immune response or reduced immunosuppressive effects of the two combinations. Yan et al. created supramolecular TP5-ICG nanofibrils by utilizing the co-assembly of immunomodulatory thymopentin (TP5, RKDVY)) and indocyanine green (ICG) for phototherapy immunotherapy of pancreatic tumors. The treatment was able to induce the proliferation of antitumor immune cells, exerting excellent effects of inhibiting tumor growth and metastasis [146]. Sensitizing cancer cells is a feasible method to reduce the toxic side effects and enhance the efficacy of photosensitizers. Considering this, Zhong et al. designed a lonidamine (LND)-conjugated peptide (LND-K) that efficiently targets the mitochondria of cancer cells. LND-K could co-assemble with photosensitizer TPPS4 into LND-K/TPPS4 nanofibers through electrostatic interactions. Thanks to the superior targeting ability of peptide, LND could be supplied progressively to both cancer cells and mitochondrial sites, which allowed it to synergistically kill cancer cells by inducing mitochondrial dysfunctions (Figure 8E-F) [147]. Targeted delivery of chemotherapeutics affords a feasible strategy for enhancing the phototherapeutic effects. Generally speaking, combined phototherapy is a desirable way to improve anti-tumor efficacy, which will certainly gain a notable place in the clinical treatment of cancer in the future.
At present, the penetration depth of light limits the phototherapy to superficial tumor, such as melanoma and breast cancer. With the development of novel photosensitizers in the far infrared region, advanced fiber optic technology and endoscopic technology, peptide-based phototherapy is gradually being applied to deep diseases, which will break the traditional depth limitations and make greater progress in various cancer therapy.
4.5 Co-assembly of immunotherapeutic agents and peptide
4.5.1. Immunotherapeutic agents-peptide co-assembly for immunotherapy
Immunotherapy is a very promising mean during cancer therapy, which exerts anti-cancer efficacy with the help of immune cells in host lymphatic tissue and anti-tumor immune cells in the tumor microenvironment. The anti-tumor immune reaction induced by immunotherapy can not only boost systemic immune monitoring, and destroy primary and disseminated tumors, but also build prolonged immune memories to prevent carcinoma recurrence. With relentless efforts, immunotherapy strategies such as immune checkpoint inhibitors-based therapy and chimeric antigen receptor (CAR) T cell therapy has been used in the clinic [148]. In addition, a considerable number of immunotherapies have entered the preclinical or clinical trials stage. Most immunotherapies are classified into the following categories: immune checkpoint inhibitor, engineered T cell therapy, lymphocyte activator, vaccine, bispecific antibody, and oncolytic virus [149-152]. Although immunotherapy has gained impressive results in some patients, it is still hindered by some problems in clinical usage. For example, due to the acquired resistance after immunotherapy, immune response rate efficiency is dissatisfactory, which hinders its clinical application.
Recently, considerable advancements in adjusting immune response and enhancing immunotherapeutic effects have been found in peptide-based assembly, which could be used as an antigen, delivery carrier, immunomodulator, or vaccine adjuvant for mono immunotherapy and combinatorial immunotherapy to treat tumors. Peptides are composed of amino acids, which are potential antigens for immunotherapy. Antigen peptides mainly include immunogenic and immune reactive peptides. Immunogenic peptides can induce an immune response, while immune reactive peptides can bind to substances to induce its immune response. Recent studies have shown that some antigenic peptides such as Q11 (Ac-QQKFQFQFEQQ-Am), have great potential in clinical anti-tumor treatment [153]. However, single molecule antigenic peptides are easy to be rapidly degraded in vivo, leading to poor antitumor immunity. On the basis of the supramolecular co-assembly technology, the assembled antigen peptide conquers the deficiency of single molecule antigen peptide, and the superiority of assembled antigen peptide includes the following aspects: (Ⅰ) Diversified structure after specific modification; (Ⅱ) Enhanced tumor accumulation; (Ⅲ) Increased stability and extended retention time in vivo; (Ⅳ) Reinforced immunogenicity and immunoreactivity. For example, Sheng et al. designed a novel cationic polypeptide (10 K-Adpgk) by modifying neoantigen peptide (Adpgk) with 10 lysines. 10 K-Adpgk was then co-assembled with CpG oligodeoxynucleotides (CpG ODN) adjuvant into nanocomplex (PCNPs) for delivering neoantigen and adjuvant. The results showed that PCNPs not only displayed a preferable capability to prompt antigen-presenting process but also boosted the activity of cytotoxic T cells, demonstrating impressive suppression of colorectal tumors [154]. Similarly, Wang and co-workers reported a nanocomplex vaccine for cancer immunotherapy. The nanocomplex vaccine was prepared by the co-assembly of the cationic peptide-conjugated epitope (Epitope-R8) and toll-like receptor 9 (TLR9) agonist CpG. The driving force of co-assembly relied on the electrostatic interactions between the oppositely charged peptide-conjugated epitope and CpG. Further results indicated that this nanocomplex vaccine could not only enhance the immunogenicity of peptides and elicit strong cytotoxic CD8+ T-cell immune responses, but also exhibit synergistic antitumor effects with immune checkpoint inhibitors [155].
In addition, peptides-drug co-assembled nanostructures can be developed for the delivery of immunotherapeutic agents. Cyclic diguanylate monophosphate (c-di-GMP) is a negatively charged stimulating factor of the interferon gene (STING) agonist with poor membrane permeability and bioavailability, which result in unfavorable therapeutic efficacy. To overcome this problem, Zeng et al. prepared a nanocomposite named c-di-GMP-PNT by co-assembling of Ac-KLVFFAL-NH2 (KL-7) and c-di-GMP, which demonstrated enhanced immune responses and preferred anti-tumor effect [156]. Liu et al. described a GSH-responsive peptide derivative (Fbp-GDFDFDYD(E, S, or K)-ss-ERGD), which could co-assemble with antigens (endotoxin-free OVA) solution to form three different hydrogels (E-vac, S-vac, and K-vac) to deliver vaccine under the catalysis of GSH. In vivo results demonstrated that K-vac displayed the optimum production of antibodies and a more significant anti-tumor immune response among these three hydrogels. The co-assemblies could serve as vaccine delivery carriers, and the obtained immune effects could be tuned by changing the physical and chemical properties of peptides [157]. In addition, their group also reported a Fbp-conjugated peptide derivative (Fbp-GDFDFDYDK(γE)2-NH2, abbreviated as Comp. 1) that was able to deliver protein antigen efficiently. In this approach, ovalbumin (OVA) and hepatitis B surface antigen (HBsAg) were chosen as protein antigen models, which could induce Comp. 1 to assemble into nanofibers/hydrogels (Figure 9A-C). This vaccine platform showed multifunctional antitumor effects, including enhanced an-tumor immune activation effects by boosting antigen-presenting cell functions and promoting dendritic cell maturation, and diminished immune suppression via restraining COX-2/PEG2 pathway [158]. Geng and co-workers designed a His6-metal assembly (HmA) delivery platform, which could co-assemble with various antibodies to form HmA@Abs for efficient intracellular delivery of antibodies [159].
Furthermore, peptide-based co-assemblies can kill tumor cells by regulating the immune system in two forms. Peptide assemblies exhibit anti-tumor properties by either activating anti-tumor immune cells or enhancing the effects of anti-tumor immunity. On the other hand, they have the potential to reduce the immunosuppressive effects and alter the microenvironments of tumors. An instance of this can be seen in the work by the Yan group, where they outlined a hydrogel co-assembled through electrostatic interactions batween poly-L-Lys (PLL) and Fmoc-FF. The finding demonstrated that this helical fibrous hydrogel could activate T cell response and effectively inhibit tumor growth (Figure 9D) [160].
4.5.2. Immunotherapeutic agents-peptide co-assembly for combined immunotherapy
The combination of immunotherapy and other therapies, such as photodynamic therapy and chemotherapy, could greatly boost the immune response and improve the synergistic anti-tumor effects, which has drawn extensive attention. For example, Wang et al. constructed a co-assembled peptide vaccine by utilizing the co-assembly of two antigen epitope-conjugated peptides (ECPs), which showed enhanced anticancer T-cell responses. The combination of PD-L1/PD-1 inhibitor and IDO inhibitor showed an enhanced antitumor immunity [161]. In this context, Nie et al described a multifunctional peptide derivative (DEAP-DPPA-1) consisting of isothiocyanate, MMP-metalloproteinase-2 responsive segment and PD-L1. The authors discovered that the micelle-like nanoparticles formed by the co-assembly of DEAP-DPPA-1 and NLG919 exhibited controlled drug release properties as a result of their dual-responsiveness in the tumor microenvironment (Figure 9E) [162]. This superiority endowed the system with enhanced antitumor immune response and few side effects. Wang et al. utilized a functionalized amphiphilic peptide (Ac-CNYSKPTDRQYHFK(C18)GGPAK(C18) GADYKPITVKVN-NH2, PCP) for the co-delivery of Dox and R848 for combination immunotherapy. The combined assembling of PCP, Dox, and R848 facilitated the development of multi-agent prodrug (PCP@R848/DOX) that not only induces a immunogenic cell death (ICD), but also stimulates cytotoxic T lymphocytes, resulting in powerful synergistic anti-tumor immune responses, as per Yu et al.'s findings [163]. Yu et al. designed three different peptides (AmpF, AmpFY, AmpFC919) based on pentapeptide FF-AmpF-FF(AmpF), all of which contained a central 4-aminoproline (AmpF) residue and two diphenylalanine (FF) fragments on both sides. AmpF was conjugated respectively with tyrosine and indoleamine 2, 3-dioxygenase (IDO) inhibitor (NLG919), AmpFY and AmpFC919 could be obtained. Once mixing AmpF, AmpFY, and AmpFC919, the co-assembled therapeutics AmpFY9 with pH shift nanostructures would be formed. In vivo assays revealed that AmpFY9 exhibited efficient delivery of NLG919 due to the augmented retention effects caused by pH-responsive morphological transformation. Interestingly, the additional γ-ray radiation would not only augment tumor immunogenicity but also stimulate immunity cytokine to inhibit the negative immune cells [164].
Overall, peptide-based co-assembly has made huge progress in remodeling tumor microenvironment, activating immune cells through ICD and developing cancer vaccines, which would expand the applicable scope of immunotherapy. With the emergence of immunotherapy combinations and artificial immune cells, the peptide-based co-assembly immunotherapy will eventually be more successful in readicating primary and metastatic tumor.
Immunotherapeutic agents-peptide co-assembly for immunotherapy and combined immunotherapy. (A) Schematic illustration of the preparation of OVA-induced co-assembled hydrogel vaccine. (B) Chemical structure of Comp.1. (C) Optical images of co-assembled hydrogel vaccine after addition OVA into Comp.1 solution. Adapted with permission from [158], copyright 2020, Ivyspring International. (D) Schematic illustration of multicomponent Fmoc-FF/PLL-SH hydrogel nanoparticles, formed by co-assembling Fmoc-FF and PLL-SH to regulate immunosuppressive tumor microenvironment. Adapted with permission from [160], copyright 2017, American Chemical Society. (E) Schematic illustration of the co-assembled peptide-based treatment integrated by peptide DEAP-DPPA-1 and NLG919 for inhibiting Trp metabolism and blocking PD-L1against cancer cells. Adapted with permission from [162], copyright 2018, American Chemical Society.
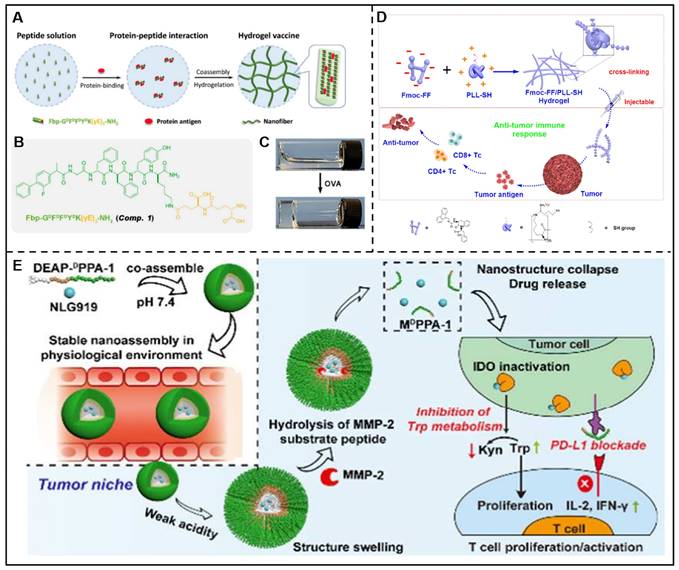
In a nutshell, peptide-based co-assembly has exhibited favorable prospects for application in chemotherapy, phototherapy, gene therapy, immunotherapy, radiotherapy, and combination therapy. As is known to all, some common functional groups found in peptide structures, such as carboxyl, hydroxyl, amino, etc, have the potential to interact with drugs via non-covalent interaction. The assembled peptides suitable for different drugs or certain anticancer drug should be adjusted according to different situations. For example, cisplatin is a common anticancer drug that could chelate with carboxyl groups in peptides. Thus, amino acids rich in carboxyl groups, such as glutamic acid, and aspartic acid were chosen as vital components of peptide segments. Some research groups represented by Yang et al. have synthesized NapFFYERGD, HCPT-FFERGD and Rh-GFFYERGD for co-assembling with cisplatin [103-105]. In a word, when developing a peptide for a certain drug, it is necessary to incorporate the assembly sequence and appropriate functional sequence in the peptide in addition to introducing the specific sequence for interacting with the drugs. This process, which peptide co-assembles with drugs to form nanostructures with different morphologies, could be adjusted both in vitro and in situ. The drugs and peptides can co-assemble to speed up the assembly process, resulting in lower in MGC and CAC values, denser nanofibers, and stronger mechanical properties. In this co-assembled system, the drug release rate could be controlled flexibly by adjusting the ratio of peptides and drugs. Generally speaking, the drug can always exhibit sustained or controlled release profiles. The multifunctionality of peptides grants the multifaceted nature of the co-assembled system. For example, the conjugation of targeted groups could significantly enhance drug enrichment in tumors and result in stronger anti-cancer effects. The drug stability in the co-assembled system can be greatly improved by the incorporation of special functional groups. Besides, dug-peptide conjugates also could co-assemble with another drug to form nanostructures, achieving synergistic anticancer effects.
5. Conclusion and Perspective
This article summarized the research progress of peptide-based co-assembly in chemotherapy, phototherapy, gene therapy, immunotherapy, radiotherapy, and combination therapy. The co-assembly approach ensured a stable drug delivery system with elevated drug loading, tuned drug release profile, improved drug stability and enhanced targeting ability of the drug. In addition, peptide-based co-assembly has achieved remarkable progress in not only the improved therapeutic effects of tumor treatment but also the reduced toxic and side effects, which has been considered as one of the potential tumor therapeutic modalities.
Although significant advances have been achieved, these stated co-assembled delivery systems are still in the pre-clinical phases. The clinical application of peptide-based co-assembly is still challenged by several potential problems. To begin with, the stability of the co-assembled delivery system needs to be improved to reduce drug leakage and prolong systemic circulation time. The current strategies, such as PEGylation of peptides, utilization of D-type amino acids, or construction of composite peptide materials, have been considered desirable methods for improving the stability of peptides. Second, the pharmacokinetics of the co-assembled delivery system needs to be fully exploited by using unified quantitative standard methods. Besides, because of the heterogeneity of tumors, it should be an effective strategy for tumor therapy to use multiple therapeutic agents. What's more, to monitor the cellular uptake and the changes in tumor tissue, it is necessary to design novel integrated diagnosis and treatment models.
In addition, the lack of in-depth exploration of the precise mechanism of assembled nanostructures is still one of the main obstacles hindering the optimum design of the co-assembled delivery system. How to optimize the proportion of peptides and drugs to obtain co-assembled nanostructures with excellent stability and superior therapeutic effects is a major problem to be solved. With the development of computer simulation technology and supramolecular chemistry, more and more novel co-assembled delivery systems with controllable structures and sophisticated functions will be explored. We expect that the peptide-based co-assembly delivery system will serve as a bridge between nanotechnology and biotechnology, and open the door to innovative clinical uses for nanomedicine, playing a role in, not only anticancer, but also antibacterial, anti-inflammatory, and other aspects.
Abbreviations
Nap: naphthylacetic acid; ZFF: carboxybenzyl-protected diphenylalanine; Fmoc: 9-Fluorenylmethoxycarbonyl; Boc: tert-butoxycarbonyl; PA: Peptide amphiphiles; R: arginine; A: alanine; D: aspartic acid; THP: pirarubicin; DOX: doxorubicin; MTX: methotrexate; MD: molecular dynamics; Lev: levofloxacin; CCM: curcumin; GSH: glutathione; IDM: indomethacin; YSV: tyroservatide; HCPT: hydroxycamptothecin; IRN: irinotecan; MMP-2: matrix metalloproteinase-2; Npx: naproxen; NIA: 2-(2-nitroimidazol-1-yl) acetic acid; DCs: dendritic cells; TLR: toll-like receptor; siRNA: small interfering RNA; pDNA: plasmid DNA; Apt: AS1411 aptamer; TPE: tetraphenylethene; 1MT: 1-methyl-DL-tryptophan; PTT: photothermal therapy; PDT: photodynamic therapy; PTCAs: photothermal conversion agents; ALP: alkaline phosphatase; BV: biliverdin; Ce6: chlorine6; THPP: 5,10,15,20-Tetrakis (4-hydroxyphenyl) porphyrin; ICG: indocyanine green; LND: lonidamine; OVA: ovalbumin; HBsAg: hepatitis B surface antigen; PLL: poly-L-Lys; ECPs: epitope-conjugated peptides; ICD: immunogenic cell death.
Acknowledgements
This work was supported by Natural Science Foundation of Henan Province (Grant No. 232300421337), National Natural Science Foundation of China (Grant No. 82274114).
Author contributions
C. Wu, X. Pang, ZQ. Zhang and YB. Guan contributed to the conception of this review. C. Wu and MM. Wang analyzed literature and wrote the manuscript. JP. Sun, XL. Zhu, GZ. Liu made contributions to draw diagrams. YY. Jia and YH. Zhu completed revising the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Mahvi DA, Liu R, Grinstaff MW, Colson YL, Raut CP. Local cancer recurrence: The realities, challenges, and opportunities for new therapies. CA Cancer J Clin. 2018;68:488-505
2. Byrd DR, Brierley JD, Baker TP, Sullivan DC, Gress DM. Current and future cancer staging after neoadjuvant treatment for solid tumors. CA Cancer J Clin. 2021;71:140-8
3. Youn YS, Bae YH. Perspectives on the past, present, and future of cancer nanomedicine. Adv Drug Deliv Rev. 2018;130:3-11
4. Hossen S, Hossain MK, Basher MK, Mia MNH, Rahman MT, Uddin MJ. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J Adv Res. 2019;15:1-18
5. Mohammapdour R, Ghandehari H. Mechanisms of immune response to inorganic nanoparticles and their degradation products. Adv Drug Deliv Rev. 2022;180:114022
6. Chambre L, Martín-Moldes Z, Parker RN, Kaplan DL. Bioengineered elastin- and silk-biomaterials for drug and gene delivery. Adv Drug Deliv Rev. 2020;160:186-98
7. Braatz D, Cherri M, Tully M, Dimde M, Ma G, Mohammadifar E. et al. Chemical approaches to synthetic drug delivery systems for systemic applications. Angew Chem Int Ed Engl. 2022;61:e202203942
8. Qi GB, Gao YJ, Wang L, Wang H. Self-assembled peptide-based nanomaterials for biomedical imaging and therapy. Adv Mater. 2018;30:1703444
9. Li T, Lu XM, Zhang MR, Hu K, Li Z. Peptide-based nanomaterials: Self-assembly, properties and applications. Bioact Mater. 2022;11:268-82
10. Stupp SI. On supramolecular self-assembly: Interview with samuel stupp. Adv Mater. 2020;32:1906741
11. Levin A, Hakala TA, Schnaider L, Bernardes GJL, Gazit E, Knowles TPJ. Biomimetic peptide self-assembly for functional materials. Nat Rev Chem. 2020;4:615-34
12. Shen Z, Guo Z, Zhou L, Wang Y, Zhang J, Hu J. et al. Biomembrane induced in situ self-assembly of peptide with enhanced antimicrobial activity. Biomater Sci. 2020;8:2031-9
13. Feng Z, Wang H, Chen X, Xu B. Self-assembling ability determines the activity of enzyme-instructed self-assembly for inhibiting cancer cells. J Am Chem Soc. 2017;139:15377-84
14. Zhan J, Cai Y, He S, Wang L, Yang Z. Tandem molecular self-assembly in liver cancer cells. Angew Chem Int Ed Engl. 2018;57:1813-6
15. Gray VP, Amelung CD, Duti IJ, Laudermilch EG, Letteri RA, Lampe KJ. Biomaterials via peptide assembly: Design, characterization, and application in tissue engineering. Acta Biomater. 2022;140:43-75
16. Peng F, Chen Y, Liu J, Xing Z, Fan J, Zhang W. et al. Facile design of gemini surfactant-like peptide for hydrophobic drug delivery and antimicrobial activity. J Colloid Interface Sci. 2021;591:314-25
17. Hu Y, Wang Y, Deng J, Ding X, Lin D, Shi H. et al. Enzyme-instructed self-assembly of peptide-drug conjugates in tear fluids for ocular drug delivery. J Control Release. 2022;344:261-71
18. Kumar VB, Ozguney B, Vlachou A, Chen Y, Gazit E, Tamamis P. Peptide self-assembled nanocarriers for cancer drug delivery. J Phys Chem B. 2023;127:1857-71
19. Zhan J, Wang Y, Ma S, Qin Q, Wang L, Cai Y. et al. Organelle-inspired supramolecular nanomedicine to precisely abolish liver tumor growth and metastasis. Bioact Mater. 2022;9:120-33
20. Yu X, Wang H, Liu X, Huang L, Song N, Song Y. et al. Assembling synergistic peptide-drug conjugates for dual-targeted treatment of cancer metastasis. Nano Today. 2022;46:101594
21. Chen Z, Zhang P, Cheetham AG, Moon JH, Moxley JW, Lin Ya. et al. Controlled release of free doxorubicin from peptide-drug conjugates by drug loading. J Control Release. 2014;191:123-30
22. Gao G, Sun X, Liu X, Jiang YW, Tang R, Guo Y. et al. Intracellular nanoparticle formation and hydroxychloroquine release for autophagy-inhibited mild-temperature photothermal therapy for tumors. Adv Funct Mater. 2021;31:2102832
23. Wang J, Hu S, Mao W, Xiang J, Zhou Z, Liu X. et al. Assemblies of peptide-cytotoxin conjugates for tumor-homing chemotherapy. Adv Funct Mater. 2019;29:1807446
24. Liang Y, Li S, Wang X, Zhang Y, Sun Y, Wang Y. et al. A comparative study of the antitumor efficacy of peptide-doxorubicin conjugates with different linkers. J Control Release. 2018;275:129-41
25. Yang J, An HW, Wang H. Self-assembled peptide drug delivery systems. ACS Appl Bio Mater. 2021;4:24-46
26. Hu X, Liao M, Gong H, Zhang L, Cox H, Waigh TA. et al. Recent advances in short peptide self-assembly: from rational design to novel applications. Curr Opin Colloid Interface Sci. 2020;45:1-13
27. Li W, Wang D, Shi X, Li J, Ma Y, Wang Y. et al. A siRNA-induced peptide co-assembly system as a peptide-based siRNA nanocarrier for cancer therapy. Mater Horiz. 2018;5:745-52
28. Jeena MT, Jeong K, Go EM, Cho Y, Lee S, Jin S. et al. Heterochiral assembly of amphiphilic peptides inside the mitochondria for supramolecular cancer therapeutics. ACS Nano. 2019;13:11022-33
29. Chen H, Guan X, Liu Q, Yang L, Guo J, Gao F. et al. Co-assembled nanocarriers of de novo thiol-activated hydrogen sulfide donors with an RGDFF pentapeptide for targeted therapy of non-small-cell lung cancer. ACS Appl Mater Interfaces. 2022;14:53475-90
30. Li Q, Jia Y, Dai L, Yang Y, Li J. Controlled rod nanostructured assembly of diphenylalanine and their optical waveguide properties. ACS Nano. 2015;9:2689-95
31. Reches M, Gazit E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science. 2003;300:625-7
32. Zaguri D, Zimmermann MR, Meisl G, Levin A, Rencus-Lazar S, Knowles TPJ. et al. Kinetic and thermodynamic driving factors in the assembly of phenylalanine-based modules. ACS Nano. 2021;15:18305-11
33. Frederix PWJM, Scott GG, Abul-Haija YM, Kalafatovic D, Pappas CG, Javid N. et al. Exploring the sequence space for (tri-)peptide self-assembly to design and discover new hydrogels. Nat Chem. 2015;7:30-7
34. Gil AM, Casanovas J, Mayans E, Jiménez AI, Puiggalí J, Alemán C. Heterochirality restricts the self-assembly of phenylalanine dipeptides capped with highly aromatic groups. J Phys Chem B. 2020;124:5913-8
35. Yang D, He H, Kim BJ, Xu B. Peptide assemblies mimicking chaperones for protein trafficking. Bioconjug Chem. 2021;32:502-6
36. Baptista RMF, Lopes PE, Rodrigues ARO, Cerca N, Belsley MS, de Matos Gomes E. Self-assembly of boc-p-nitro-l-phenylalanyl-p-nitro-l-phenylalanine and boc-l-phenylalanyl-l-tyrosine in solution and into piezoelectric electrospun fibers. Mater Adv. 2022;3:2934-44
37. Feng K, Ma C, Liu Y, Yang X, Yang Z, Chen Y. et al. Encapsulation of LXR ligand by D-Nap-GFFY hydrogel enhances anti-tumorigenic actions of LXR and removes LXR-induced lipogenesis. Theranostics. 2021;11:2634-54
38. Ji W, Tang Y, Makam P, Yao Y, Jiao R, Cai K. et al. Expanding the structural diversity and functional scope of diphenylalanine-based peptide architectures by hierarchical coassembly. J Am Chem Soc. 2021;143:17633-45
39. Xiong Q, Jiang Y, Cai X, Yang F, Li Z, Han W. Conformation dependence of diphenylalanine self-assembly structures and dynamics: Insights from hybrid-resolution simulations. ACS Nano. 2019;13:4455-68
40. Wang Y, Geng Q, Zhang Y, Adler-Abramovich L, Fan X, Mei D. et al. Fmoc-diphenylalanine gelating nanoarchitectonics: A simplistic peptide self-assembly to meet complex applications. J Colloid Interface Sci. 2023;636:113-33
41. Wang J, Liu K, Xing R, Yan X. Peptide self-assembly: thermodynamics and kinetics. Chem Soc Rev. 2016;45:5589-604
42. Guo C, Luo Y, Zhou R, Wei G. Probing the self-assembly mechanism of diphenylalanine-based peptide nanovesicles and nanotubes. ACS Nano. 2012;6:3907-18
43. Wang Y, Qi W, Huang R, Yang X, Wang M, Su R. et al. Rational design of chiral nanostructures from self-assembly of a ferrocene-modified dipeptide. J Am Chem Soc. 2015;137:7869-80
44. Li Q, Jia Y, Dai L, Yang Y, Li J. Controlled rod nanostructured assembly of diphenylalanine and their optical waveguide properties. ACS Nano. 2015;9:2689-95
45. Liu M, Wang Z, Feng D, Shang Y, Li X, Liu J. et al. An insulin-inspired supramolecular hydrogel for prevention of type 1 diabetes. Adv Sci. 2021;8:2003599
46. Cheng B, Yan Y, Qi J, Deng L, Shao ZW, Zhang KQ. et al. Cooperative assembly of a peptide gelator and silk fibroin afford an injectable hydrogel for tissue engineering. ACS Appl Mater Interfaces. 2018;10:12474-84
47. Sun M, Zhang X, Gao Z, Liu T, Luo C, Zhao Y. et al. Probing a dipeptide-based supramolecular assembly as an efficient camptothecin delivering carrier for cancer therapy: computational simulations and experimental validations. Nanoscale. 2019;11:3864-76
48. Wu A, Guo Y, Li X, Li Q, Chen G, Zang H. et al. Schiff base nanoarchitectonics for supramolecular assembly of dipeptide as drug carriers. J Colloid Interface Sci. 2023;630:161-9
49. Chauhan N, Singh Y. Self-assembled Fmoc-arg-phe-phe peptide gels with highly potent bactericidal activities. ACS Biomater Sci Eng. 2020;6:5507-18
50. Zhao Y, Yang W, Chen C, Wang J, Zhang L, Xu H. Rational design and self-assembly of short amphiphilic peptides and applications. Curr Opin Colloid Interface Sci. 2018;35:112-23
51. Kornmueller K, Lehofer B, Leitinger G, Amenitsch H, Prassl R. Peptide self-assembly into lamellar phases and the formation of lipid-peptide nanostructures. Nano Res. 2018;11:913-28
52. Li J, Wang J, Zhao Y, Zhou P, Carter J, Li Z. et al. Surfactant-like peptides: From molecular design to controllable self-assembly with applications. Coord Chem Rev. 2020;421:213418
53. Zhao C, Chen H, Wang F, Zhang X. Amphiphilic self-assembly peptides: Rational strategies to design and delivery for drugs in biomedical applications. Colloids Surf B Biointerfaces. 2021;208:112040
54. Gong Z, Shi Y, Tan H, Wang L, Gao Z, Lian B. et al. Plasma amine oxidase-induced nanoparticle-to-nanofiber geometric transformation of an amphiphilic peptide for drug encapsulation and enhanced bactericidal activity. ACS Appl Mater Interfaces. 2020;12:4323-32
55. Wang D, Zhang X, Li H, Luan Y, Wei G, Wang J. Anticancer properties of lipidated peptide drug supramolecular self-assemblies with enhanced stability. ACS Appl Bio Mater. 2019;2:5995-6003
56. Sangji MH, Sai H, Chin SM, Lee SR, R Sasselli I, Palmer LC. et al. Supramolecular interactions and morphology of self-assembling peptide amphiphile nanostructures. Nano Lett. 2021;21:6146-55
57. Jiao D, Geng J, Loh XJ, Das D, Lee T-C, Scherman OA. Supramolecular peptide amphiphile vesicles through host-guest complexation. Angew Chem Int Ed Engl. 2012;51:9633-7
58. Dowari P, Das S, Pramanik B, Das D. pH clock instructed transient supramolecular peptide amphiphile and its vesicular assembly. Chem Commun. 2019;55:14119-22
59. Liang C, Zhang L, Zhao W, Xu L, Chen Y, Long J. et al. Supramolecular nanofibers of drug-peptide amphiphile and affibody suppress HER2+ tumor growth. Adv Healthc Mater. 2018;7:1800899
60. Gong Z, Liu X, Dong J, Zhang W, Jiang Y, Zhang J. et al. Transition from vesicles to nanofibres in the enzymatic self-assemblies of an amphiphilic peptide as an antitumour drug carrier. Nanoscale. 2019;11:15479-86
61. Wychowaniec JK, Patel R, Leach J, Mathomes R, Chhabria V, Patil-Sen Y. et al. Aromatic stacking facilitated self-assembly of ultrashort ionic complementary peptide sequence: β-sheet nanofibers with remarkable gelation and interfacial properties. Biomacromolecules. 2020;21:2670-80
62. Pal VK, Jain R, Roy S. Tuning the supramolecular structure and function of collagen mimetic ionic complementary peptides via electrostatic interactions. Langmuir. 2020;36:1003-13
63. Yan J, Wang Y, Li X, Guo D, Zhou Z, Bai G. et al. A bionic nano-band-aid constructed by the three-stage self-assembly of peptides for rapid liver hemostasis. Nano Lett. 2021;21:7166-74
64. Wan C, Sun Y, Hu Y, Huang J, Lu L, Gao Y. et al. Peptide hydrogels loaded with irradiated tumor cell secretions enhance cancer immunotherapy. Nano Today. 2021;41:101323
65. Yang P, Song H, Qin Y, Huang P, Zhang C, Kong D. et al. Engineering dendritic-cell-based vaccines and PD-1 blockade in self-assembled peptide nanofibrous hydrogel to amplify antitumor T-Cell immunity. Nano Lett. 2018;18:4377-85
66. De Santis P, Morosetti S, Rizzo R. Conformational analysis of regular enantiomeric sequences. Macromolecules. 1974;7:52-8
67. Ghadiri MR, Granja JR, Milligan RA, McRee DE, Khazanovich N. Self-assembling organic nanotubes based on a cyclic peptide architecture. Nature. 1993;366:324-7
68. Carlini AS, Gaetani R, Braden RL, Luo C, Christman KL, Gianneschi NC. Enzyme-responsive progelator cyclic peptides for minimally invasive delivery to the heart post-myocardial infarction. Nat Commun. 2019;10:1735
69. Insua I, Montenegro J. 1D to 2D self assembly of cyclic peptides. J Am Chem Soc. 2020;142:300-7
70. Calvelo M, Lynch CI, Granja JR, Sansom MSP, Garcia-Fandiño R. Effect of water models on transmembrane self-assembled cyclic peptide nanotubes. ACS Nano. 2021;15:7053-64
71. Zhai W, Zhou X, Wang H, Li W, Chen G, Sui X. et al. A novel cyclic peptide targeting LAG-3 for cancer immunotherapy by activating antigen-specific CD8+ T cell responses. Acta Pharm Sin B. 2020;10:1047-60
72. Yang DS, Yang YH, Zhou Y, Yu LL, Wang RH, Di B. et al. A redox-triggered bispecific supramolecular nanomedicine based on peptide self-assembly for high-efficacy and low-toxic cancer therapy. Adv Funct Mater. 2020;30:1904969
73. Li M, Ehlers M, Schlesiger S, Zellermann E, Knauer SK, Schmuck C. Incorporation of a non-natural arginine analogue into a cyclic peptide leads to formation of positively charged nanofibers capable of gene transfection. Angew Chem Int Ed Engl. 2016;55:598-601
74. Ragozin E, Hesin A, Bazylevich A, Tuchinsky H, Bovina A, Shekhter Zahavi T. et al. New somatostatin-drug conjugates for effective targeting pancreatic cancer. Bioorg Med Chem. 2018;26:3825-36
75. Lou S, Wang X, Yu Z, Shi L. Peptide tectonics: Encoded structural complementarity dictates programmable self-assembly. Adv Sci. 2019;6:1802043
76. Wang J, Liu K, Xing R, Yan X. Peptide self-assembly: thermodynamics and kinetics. Chem Soc Rev. 2016;45:5589-604
77. Liu Y, Guo X, Zhao M, Zou C, Feng Y, Wu Y. et al. The effect and enhancement mechanism of hydrophobic interaction and electrostatic interaction on zwitterionic wormlike micelles. Colloids Surf A Physicochem Eng Asp. 2022;648:129424
78. Xiong Q, Stupp SI, Schatz GC. Molecular insight into the β-sheet twist and related morphology of self-assembled peptide amphiphile ribbons. J Phys Chem Lett. 2021;12:11238-44
79. Sadatmousavi P, Chen P. Self/co-assembling peptide, ear8-ii, as a potential carrier for a hydrophobic anticancer drug pirarubicin (thp)—characterization and in-vitro delivery. Int J Mol Sci. 2013;14:23315-29
80. Basavalingappa V, Bera S, Xue B, O'Donnell J, Guerin S, Cazade P-A. et al. Diphenylalanine-derivative peptide assemblies with increased aromaticity exhibit metal-like rigidity and high piezoelectricity. ACS Nano. 2020;14:7025-37
81. Abraham BL, Mensah SG, Gwinnell BR, Nilsson BL. Side-chain halogen effects on self-assembly and hydrogelation of cationic phenylalanine derivatives. Soft Matter. 2022;18:5999-6008
82. Chen C, Chen J, Yu Q, Zhang J, Niu X, Hao L. et al. Effects of salts on the self-assembly behavior and antibacterial activity of a surfactant-like peptide. Soft Matter. 2020;16:9758-68
83. Kaur H, Sharma P, Patel N, Pal VK, Roy S. Accessing highly tunable nanostructured hydrogels in a short ionic complementary peptide sequence via pH trigger. Langmuir. 2020;36:12107-20
84. Sahoo JK, VandenBerg MA, Ruiz Bello EE, Nazareth CD, Webber MJ. Electrostatic-driven self-sorting and nanostructure speciation in self-assembling tetrapeptides. Nanoscale. 2019;11:16534-43
85. Mei L, Xu K, Zhai Z, He S, Zhu T, Zhong W. Doxorubicin-reinforced supramolecular hydrogels of RGD-derived peptide conjugates for pH-responsive drug delivery. Org Biomol Chem. 2019;17:3853-60
86. Rho JY, Cox H, Mansfield EDH, Ellacott SH, Peltier R, Brendel JC. et al. Dual self-assembly of supramolecular peptide nanotubes to provide stabilisation in water. Nat Commun. 2019;10:4708
87. Wang J, Liu K, Yan L, Wang A, Bai S, Yan X. Trace solvent as a predominant factor to tune dipeptide self-assembly. ACS Nano. 2016;10:2138-43
88. Cheng H, Cheng YJ, Bhasin S, Zhu JY, Xu XD, Zhuo RX. et al. Complementary hydrogen bonding interaction triggered co-assembly of an amphiphilic peptide and an anti-tumor drug. Chem Commun. 2015;51:6936-9
89. Zhao C, Wang Y, Shi B, Li M, Yan W, Yang H. Tailoring co-assembly loading of doxorubicin in solvent-triggering gel. J Colloid Interface Sci. 2022;626:619-28
90. Deng L, Zhao Y, Zhou P, Xu H, Wang Y. Anisotropic formation mechanism and nanomechanics for the self-assembly process of cross-β peptides. Chin Phys B. 2017;26:128701
91. Yu A, Hu Y, Ma X, Mo L, Pan M, Bi X. et al. Sequential drug release of co-assembled supramolecular hydrogel as synergistic therapy against staphylococcus aureus endophthalmitis. Chem Eng J. 2022;427:130979
92. Liu K, Xing R, Zou Q, Ma G, Möhwald H, Yan X. Simple peptide-tuned self-assembly of photosensitizers towards anticancer photodynamic therapy. Angew Chem Int Ed Engl. 2016;128:3088-91
93. Fu D, Calvo JA, Samson LD. Balancing repair and tolerance of DNA damage caused by alkylating agents. Nat Rev Cancer. 2012;12:104-20
94. Valenzuela MM, Neidigh JW, Wall NR. Antimetabolite treatment for pancreatic cancer. Chemotherapy. 2014;3:137
95. Barreca M, Stathis A, Barraja P, Bertoni F. An overview on anti-tubulin agents for the treatment of lymphoma patients. Pharmacol Ther. 2020;211:107552
96. Bax BD, Murshudov G, Maxwell A, Germe T. DNA topoisomerase inhibitors: trapping a DNA-cleaving machine in motion. J Mol Biol. 2019;431:3427-49
97. Gao Y, Shang Q, Li W, Guo W, Stojadinovic A, Mannion C. et al. Antibiotics for cancer treatment: A double-edged sword. J Cancer. 2020;11:5135-49
98. Dai Y, Jiang Z, Li J, Wang M, Liu C, Qi W. et al. Co-assembly of curcumin and a cystine bridged peptide to construct tumor-responsive nano-micelles for efficient chemotherapy. J Mater Chem B. 2020;8:1944-51
99. Ji T, Zhao Y, Ding Y, Wang J, Zhao R, Lang J. et al. Transformable peptide nanocarriers for expeditious drug release and effective cancer therapy via cancer-associated fibroblast activation. Angew Chem Int Ed Engl. 2016;55:1050-5
100. Li S, Chen X, Chen H, Peng J, Yang X. Small peptide-doxorubicin co-assembly for synergistic cancer therapy. Molecules. 2020;25:484
101. Mei L, He S, Liu Z, Xu K, Zhong W. Co-assembled supramolecular hydrogels of doxorubicin and indomethacin-derived peptide conjugates for synergistic inhibition of cancer cell growth. Chem Commun. 2019;55:4411-4
102. Liu J, Wu C, Dai G, Feng F, Chi Y, Xu K. et al. Molecular self-assembly of a tyroservatide-derived octapeptide and hydroxycamptothecin for enhanced therapeutic efficacy. Nanoscale. 2021;13:5094-102
103. Wu C, Liu J, Zhai Z, Yang L, Tang X, Zhao L. et al. Double-crosslinked nanocomposite hydrogels for temporal control of drug dosing in combination therapy. Acta Biomater. 2020;106:278-88
104. Cai Y, Shen H, Zhan J, Lin M, Dai L, Ren C. et al. Supramolecular “trojan horse” for nuclear delivery of dual anticancer drugs. J Am Chem Soc. 2017;139:2876-9
105. Xu T, Liang C, Zheng D, Yan X, Chen Y, Chen Y. et al. Nuclear delivery of dual anticancer drug-based nanomedicine constructed by cisplatinum-induced peptide self-assembly. Nanoscale. 2020;12:15275-82
106. Anzela A, Min M, Knesl M, Buddle N, Azzopardi M, Hooshmand R. et al. Concurrent carbogen and nicotinamide with radiation therapy in muscle invasive bladder cancer: A report on feasibility in the australian setting. J Med Imaging Radiat Oncol. 2021;65:768-77
107. Ogita M, Kawamori J, Yamashita H, Nakagawa K. Palliative radiotherapy for gross hematuria in patients with advanced cancer. Sci Rep. 2021;11:9533
108. Farhood B, Mortezaee K, Haghi-Aminjan H, Khanlarkhani N, Salehi E, Nashtaei MS. et al. A systematic review of radiation-induced testicular toxicities following radiotherapy for prostate cancer. J Cell Physiol. 2019;234:14828-37
109. Hunt AA, Choudhury KR, Nukala V, Nolan MW, Ahmad A, Ashcraft KA. et al. Risk of erectile dysfunction after modern radiotherapy for intact prostate cancer. Prostate Cancer Prostatic Dis. 2021;24:128-34
110. Salvestrini V, Iorio GC, Borghetti P, De Felice F, Greco C, Nardone V. et al. The impact of modern radiotherapy on long-term cardiac sequelae in breast cancer survivor: a focus on deep inspiration breath-hold (DIBH) technique. J Cancer Res Clin Oncol. 2022;148:409-17
111. Xu H, Wang T, Yang C, Li X, Liu G, Yang Z. et al. Supramolecular nanofibers of curcumin for highly amplified radiosensitization of colorectal cancers to ionizing radiation. Adv Funct Mater. 2018;28:1707140
112. Liu J, Zhang Y, Li Q, Feng Z, Huang P, Wang W. et al. Development of injectable thermosensitive polypeptide hydrogel as facile radioisotope and radiosensitizer hotspot for synergistic brachytherapy. Acta Biomater. 2020;114:133-45
113. Yang C, Mu G, Zhang Y, Gao Y, Zhang W, Liu J. et al. Supramolecular nitric oxide depot for hypoxic tumor vessel normalization and radiosensitization. Adv Mater. 2022;34:2202625
114. Wang Q, Hou X, Gao J, Ren C, Guo Q, Fan H. et al. A coassembled peptide hydrogel boosts the radiosensitization of cisplatin. Chem Commun. 2020;56:13017-20
115. Wang H, Wang Z, Chen W, Wang W, Shi W, Chen J. et al. Self-assembly of photosensitive and radiotherapeutic peptide for combined photodynamic-radio cancer therapy with intracellular delivery of miRNA-139-5p. Bioorg Med Chem. 2021;44:116305
116. Chandra RA, Wilhite TJ, Balboni TA, Alexander BM, Spektor A, Ott PA. et al. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology. 2015;4:e1046028
117. Zhu X, Wang X, Li B, Zhang Y, Chen Y, Zhang W. et al. A Three-in-one assembled nanoparticle containing peptide-radio-sensitizer conjugate and TLR7/8 Agonist can initiate the cancer-immunity cycle to trigger antitumor immune response. Small. 2022;18:2107001
118. Chen H, Guan X, Liu Q, Yang L, Guo J, Gao F. et al. Co-assembled nanocarriers of de novo thiol-activated hydrogen sulfide donors with an RGDFF pentapeptide for targeted therapy of non-small-cell lung cancer. ACS Appl Mater Interfaces. 2022;14:53475-90
119. Parsel SM, Grandis JR, Thomas SM. Nucleic acid targeting: towards personalized therapy for head and neck cancer. Oncogene. 2016;35:3217-26
120. Hu B, Zhong L, Weng Y, Peng L, Huang Y, Zhao Y. et al. Therapeutic siRNA: state of the art. Signal Transduct Target Ther. 2020;5:101
121. Charbe NB, Amnerkar ND, Ramesh B, Tambuwala MM, Bakshi HA, Aljabali AAA. et al. Small interfering RNA for cancer treatment: overcoming hurdles in delivery. Acta Pharm Sin B. 2020;10:2075-109
122. Zuckerman JE, Davis ME. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat Rev Drug Discov. 2015;14:843-56
123. Vaissière A, Aldrian G, Konate K, Lindberg MF, Jourdan C, Telmar A. et al. A retro-inverso cell-penetrating peptide for siRNA delivery. J Nanobiotechnology. 2017;15:34
124. Dehghani S, Alibolandi M, Tehranizadeh ZA, Oskuee RK, Nosrati R, Soltani F. et al. Self-assembly of an aptamer-decorated chimeric peptide nanocarrier for targeted cancer gene delivery. Colloids Surf B Biointerfaces. 2021;208:112047
125. Zhang C, Zhang T, Jin S, Xue X, Yang X, Gong N. et al. Virus-inspired self-assembled nanofibers with aggregation-induced emission for highly efficient and visible gene delivery. ACS Appl Mater Interfaces. 2017;9:4425-32
126. Li W, Wang D, Shi X, Li J, Ma Y, Wang Y. et al. A siRNA-induced peptide co-assembly system as a peptide-based siRNA nanocarrier for cancer therapy. Mater Horiz. 2018;5:745-52
127. Ma Y, Li W, Zhou Z, Qin X, Wang D, Gao Y. et al. Peptide-aptamer coassembly nanocarrier for cancer therapy. Bioconjug Chem. 2019;30:536-40
128. Tuttolomondo M, Casella C, Hansen PL, Polo E, Herda LM, Dawson KA. et al. Human DMBT1-derived cell-penetrating peptides for intracellular siRNA delivery. Mol Ther Nucleic Acids. 2017;8:264-76
129. Al-Husaini K, Elkamel E, Han X, Chen P. Therapeutic potential of a cell penetrating peptide (CPP, NP1) mediated siRNA delivery: Evidence in 3D spheroids of colon cancer cells. Can J Chem Eng. 2020;98:1240-54
130. Pan R, Xu W, Yuan F, Chu D, Ding Y, Chen B. et al. A novel peptide for efficient siRNA delivery in vitro and therapeutics in vivo. Acta Biomater. 2015;21:74-84
131. Cheng Y, Sun C, Liu R, Yang J, Dai J, Zhai T. et al. A multifunctional peptide-conjugated AIEgen for efficient and sequential targeted gene delivery into the nucleus. Angew Chem Int Ed Engl. 2019;131:5103-7
132. Yang J, Dai J, Wang Q, Cheng Y, Guo J, Zhao Z. et al. Tumor-triggered disassembly of a multiple-agent-therapy probe for efficient cellular internalization. Angew Chem Int Ed Engl. 2020;132:20585-90
133. Li G, Gao Y, Gong C, Han Z, Qiang L, Tai Z. et al. Dual-blockade immune checkpoint for breast cancer treatment based on a tumor-penetrating peptide assembling nanoparticle. ACS Appl Mater Interfaces. 2019;11:39513-24
134. Shirata C, Kaneko J, Inagaki Y, Kokudo T, Sato M, Kiritani S. et al. Near-infrared photothermal/photodynamic therapy with indocyanine green induces apoptosis of hepatocellular carcinoma cells through oxidative stress. Sci Rep. 2017;7:13958
135. Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17:657-74
136. Huang P, Gao Y, Lin J, Hu H, Liao H-S, Yan X. et al. Tumor-specific formation of enzyme-instructed supramolecular self-assemblies as cancer theranostics. ACS Nano. 2015;9:9517-27
137. Xing R, Zou Q, Yuan C, Zhao L, Chang R, Yan X. Self-assembling endogenous biliverdin as a versatile near-infrared photothermal nanoagent for cancer theranostics. Adv Mater. 2019;31:1900822
138. Golding JP, Wardhaugh T, Patrick L, Turner M, Phillips JB, Bruce JI. et al. Targeting tumour energy metabolism potentiates the cytotoxicity of 5-aminolevulinic acid photodynamic therapy. Br J Cancer. 2013;109:976-82
139. Pham TC, Nguyen V-N, Choi Y, Lee S, Yoon J. Recent strategies to develop innovative photosensitizers for enhanced photodynamic therapy. Chem Rev. 2021;121:13454-619
140. Li J, Wang A, Ren P, Yan X, Bai S. One-step co-assembly method to fabricate photosensitive peptide nanoparticles for two-photon photodynamic therapy. Chem Commun. 2019;55:3191-4
141. Zhang H, Liu K, Li S, Xin X, Yuan S, Ma G. et al. Self-assembled minimalist multifunctional theranostic nanoplatform for magnetic resonance imaging-guided tumor photodynamic therapy. ACS Nano. 2018;12:8266-76
142. Li S, Zou Q, Li Y, Yuan C, Xing R, Yan X. Smart peptide-based supramolecular photodynamic metallo-nanodrugs designed by multicomponent coordination self-assembly. J Am Chem Soc. 2018;140:10794-802
143. Wan Y, Fu LH, Li C, Lin J, Huang P. Conquering the hypoxia limitation for photodynamic therapy. Adv Mater. 2021;33:2103978
144. Hou Y, Kuang Y, Jiang Q, Zhou S, Yu J, He Z. et al. Arginine-peptide complex-based assemblies to combat tumor hypoxia for enhanced photodynamic therapeutic effect. Nano Res. 2022;15:5183-92
145. Wan G, Cheng Y, Song J, Chen Q, Chen B, Liu Y. et al. Nucleus-targeting near-infrared nanoparticles based on TAT peptide-conjugated IR780 for photo-chemotherapy of breast cancer. Chem Eng J. 2020;380:122458
146. Li S, Zhang W, Xing R, Yuan C, Xue H, Yan X. Supramolecular nanofibrils formed by coassembly of clinically approved drugs for tumor photothermal immunotherapy. Adv Mater. 2021;33:2100595
147. Wu C, Jiao Q, Wang C, Zheng Y, Pan X, Zhong W. et al. Nanofibrillar peptide hydrogels for self-delivery of lonidamine and synergistic photodynamic therapy. Acta Biomater. 2023;155:139-53
148. Kansagra AJ, Frey NV, Bar M, Laetsch TW, Carpenter PA, Savani BN. et al. Clinical utilization of chimeric antigen receptor T-cells (CAR-T) in B-cell acute lymphoblastic leukemia (ALL)-an expert opinion from the european society for blood and marrow transplantation (EBMT) and the american society for blood and marrow transplantation (ASBMT). Bone Marrow Transplant. 2019;54:1868-80
149. Xue L, Yi Y, Xu Q, Wang L, Yang X, Zhang Y. et al. Chimeric antigen receptor T cells self-neutralizing IL6 storm in patients with hematologic malignancy. Cell Discov. 2021;7:84
150. D'Abreo N, Adams S. Immune-checkpoint inhibition for metastatic triple-negative breast cancer: safety first? Nat Rev Clin Oncol. 2019;16:399-400
151. Korman AJ, Garrett-Thomson SC, Lonberg N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat Rev Drug Discov. 2022;21:509-28
152. Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14:642-62
153. Hudalla GA, Modica JA, Tian YF, Rudra JS, Chong AS, Sun T. et al. A self-adjuvanting supramolecular vaccine carrying a folded protein antigen. Adv Healthc Mater. 2013;2:1114-9
154. Liang Z, Cui X, Yang L, Hu Q, Li D, Zhang X. et al. Co-assembled nanocomplexes of peptide neoantigen Adpgk and Toll-like receptor 9 agonist CpG ODN for efficient colorectal cancer immunotherapy. Int J Pharm. 2021;608:121091
155. Shi X, Song H, Wang C, Zhang C, Huang P, Kong D. et al. Co-assembled and self-delivered epitope/CpG nanocomplex vaccine augments peptide immunogenicity for cancer immunotherapy. Chem Eng J. 2020;399:125854
156. Zhang Z, Liu J, Xiao M, Zhang Q, Liu Z, Liu M. et al. Peptide nanotube loaded with a STING agonist, c-di-GMP, enhance cancer immunotherapy against melanoma. Nano Res. 2023;16:5206-15
157. Wang Z, Ren C, Shang Y, Yang C, Guo Q, Chu L. et al. Co-assembled supramolecular nanofibers with tunable surface properties for efficient vaccine delivery. Front Chem. 2020;8:500
158. Wang Z, Shang Y, Tan Z, Li X, Li G, Ren C. et al. A supramolecular protein chaperone for vaccine delivery. Theranostics. 2020;10:657
159. Wei S, Zhou S, Huang W, Zan X, Geng W. Efficient delivery of antibodies intracellularly by co-assembly with hexahistidine-metal assemblies (HmA). Int J Nanomedicine. 2021;16:7449-61
160. Xing R, Li S, Zhang N, Shen G, Möhwald H, Yan X. Self-assembled injectable peptide hydrogels capable of triggering antitumor immune response. Biomacromolecules. 2017;18:3514-23
161. Su Q, Song H, Huang P, Zhang C, Yang J, Kong D. et al. Supramolecular co-assembly of self-adjuvanting nanofibrious peptide hydrogel enhances cancer vaccination by activating MyD88-dependent NF-κB signaling pathway without inflammation. Bioact Mater. 2021;6:3924-34
162. Cheng K, Ding Y, Zhao Y, Ye S, Zhao X, Zhang Y. et al. Sequentially responsive therapeutic peptide assembling nanoparticles for dual-targeted cancer immunotherapy. Nano Lett. 2018;18:3250-8
163. Sun M, Yao S, Fan L, Fang Z, Miao W, Hu Z. et al. Fibroblast activation protein-α responsive peptide assembling prodrug nanoparticles for remodeling the immunosuppressive microenvironment and boosting cancer immunotherapy. Small. 2022;18:2106296
164. Li M, Wang Z, Liu X, Song N, Song Y, Shi X. et al. Adaptable peptide-based therapeutics modulating tumor microenvironment for combinatorial radio-immunotherapy. J Control Release. 2021;340:35-47
Author contact
![]() Corresponding authors: Xin Pang (pangxin116com); Zhenqiang Zhang (zhang_zhenqiangcom); Yanbin Guan (guanyanbin96com).
Corresponding authors: Xin Pang (pangxin116com); Zhenqiang Zhang (zhang_zhenqiangcom); Yanbin Guan (guanyanbin96com).
 Global reach, higher impact
Global reach, higher impact