13.3
Impact Factor
Theranostics 2024; 14(2):451-459. doi:10.7150/thno.92487 This issue Cite
Research Paper
Long-term Nephrotoxicity after PRRT: Myth or Reality
1. CURANOSTICUM Wiesbaden-Frankfurt, Center for Advanced Radiomolecular Precision Oncology, Wiesbaden, Germany.
2. Theranostics Center for Molecular Radiotherapy and Precision Oncology, ENETS Center of Excellence, Zentralklinik Bad Berka, Bad Berka, Germany.
3. Department of Diagnostic Radiology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore.
4. Clinical Imaging Research Centre, Centre for Translational Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore.
5. Department of Nuclear Medicine, Shanghai Tenth People's Hospital, Tongji University School of Medicine, Shanghai, China.
6. Institute of Nuclear Medicine, Tongji University School of Medicine, Shanghai, China.
7. Nanomedicine Translational Research Program, NUS Center for Nanomedicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore.
8. Department of Surgery, Chemical and Biomolecular Engineering, and Biomedical Engineering, Yong Loo Lin School of Medicine and College of Design and Engineering, National University of Singapore, Singapore, Singapore.
9. Institute of Molecular and Cell Biology, Agency for Science, Technology, and Research (A*STAR), 61 Biopolis Drive, Proteos, Singapore, Singapore.
# These authors contributed equally to this work.
Abstract
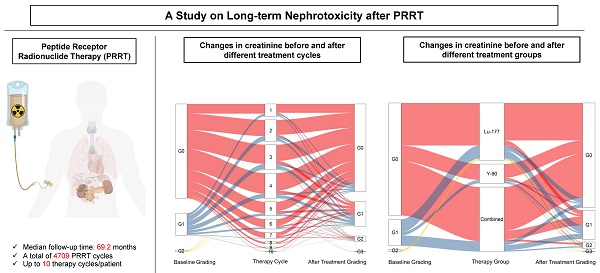
Rationale: The kidneys are commonly considered as the potential dose-limiting organ for peptide receptor radionuclide therapy (PRRT), making the risk of nephrotoxicity a primary concern. This retrospective analysis with prospective documentation and long-term follow-up aims to assess the risk of nephrotoxicity after PRRT in a large cohort of patients with neuroendocrine neoplasms (NENs) treated at our institution over the past 18 years.
Methods: A total of 1361 NEN patients treated with 1-10 cycles of 177Lu-DOTA-TOC/-NOC/-TATE, 90Y-DOTA-TOC/-NOC/-TATE, DUO-PRRT (sequential administration of 90Y- and 177Lu-), or TANDEM-PRRT (combination of 90Y- and 177Lu- on the same day concomitantly) were included in this analysis. All parameters were prospectively documented in a structured database comprising over 250 items per patient and retrospectively analyzed. Kidney function, including serum creatinine, blood urea nitrogen, cGFR, and electrolytes, was evaluated before each PRRT cycle and during follow-up. Restaging was regularly performed at 6-month intervals until death. Treatment-related adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE v.5.0).
Results: Between 2000 and 2018, a total of 5409 cycles of PRRT were administered to 1361 NEN patients. Follow-up after complete treatment was available for 1281 patients receiving 4709 cycles of PRRT, with a median follow-up time of 69.2 months (interquartile range, 32.8-110.5 months) and a maximum follow-up time of 175 months. Baseline creatinine levels were normal in 1039/1281 (81.1%) subjects, while grade 1 (G1) renal insufficiency was present in 221/1281 (17.3%) prior to PRRT. G2 was present in 19/1281 (1.5%), and G3 in 2/1281 (0.2%). After treatment, the proportion of G3/G4 grade patients only increased from 0.2% to 0.7%. Mean creatinine levels increased from a baseline of 0.90 ± 0.30 to 1.01 ± 0.57 mg/L (80.0 ± 26.7 to 89.4 ± 50.8 μmol/L) after treatment. In our main analysis cohort of 1244 patients (4576 cycles), 200 patients experienced an increase in CTCAE creatinine grade. Age, number of treatment cycles, type of radionuclides, and length of follow-up time were the main factors affecting CTCAE creatinine grading after treatment. When comparing the subgroups treated with different radionuclides, the risk of nephrotoxicity after 90Y treatment alone and the 90Y/177Lu combination group was higher than after 177Lu treatment alone. In the 90Y treatment subgroup, the two significant risk factors for an increased CTCAE creatinine grade were identified to be age (≥60) and a long follow-up time.
Conclusions: This retrospective analysis with prospective documentation in a large cohort of 1281 NEN patients receiving 4709 cycles of PRRT co-administered with renal protection, treated through the individualized approach at a single institution over 18 years, did not reveal any evidence of long-term PRRT-related renal toxicity. The results of our study suggest that with the use of proper renal protection, nephrotoxicity due to PRRT is more likely a myth than a reality.
Keywords: peptide receptor radionuclide therapy (PRRT), nephrotoxicity, long-term, lutetium-177 (177Lu), yttrium-90 (90Y), somatostatin analogs
 Global reach, higher impact
Global reach, higher impact