13.3
Impact Factor
Theranostics 2024; 14(2):480-495. doi:10.7150/thno.89592 This issue Cite
Research Paper
Cerebellar Purkinje cell firing promotes conscious recovery from anesthesia state through coordinating neuronal communications with motor cortex
1. Department of Anesthesiology, Department of Neurosurgery, Zhongnan Hospital, Wuhan University, Wuhan 430071, China.
2. Department of Anesthesiology, The Children's Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China.
3. Department of Anesthesia and Intensive Care, Peter Hung Pain Research Institute, The Chinese University of Hong Kong, Hong Kong SAR, P.R. China.
4. Key Laboratory of Magnetic Resonance in Biological Systems, State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, National Center for Magnetic Resonance in Wuhan, Wuhan Institute of Physics and Mathematics, Innovation Academy for Precision Measurement Science and Technology, Chinese Academy of Sciences-Wuhan National Laboratory for Optoelectronics, Wuhan 430071, China.
5. Department of Anesthesiology, Zhongshan Hospital, Fudan University, Shanghai, China.
6. University of Chinese Academy of Sciences, Beijing 100049, China.
7. Division of Anesthetics, Pain Medicine and Intensive Care, Department of Surgery and Cancer, Faculty of Medicine, Imperial College London, Chelsea and Westminster Hospital, London, UK.
# These authors contributed equally to this work.
Abstract
Background: The neurobiological basis of gaining consciousness from unconscious state induced by anesthetics remains unknown. This study was designed to investigate the involvement of the cerebello-thalamus-motor cortical loop mediating consciousness transitions from the loss of consciousness (LOC) induced by an inhalational anesthetic sevoflurane in mice.
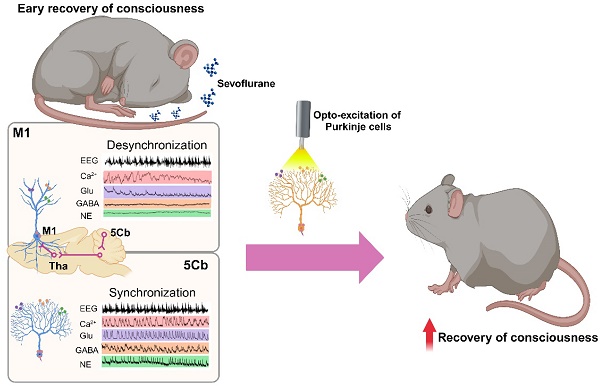
Methods: The neural tracing and fMRI together with opto-chemogenetic manipulation were used to investigate the potential link among cerebello-thalamus-motor cortical brain regions. The fiber photometry of calcium and neurotransmitters, including glutamate (Glu), γ-aminobutyric acid (GABA) and norepinephrine (NE), were monitored from the motor cortex (M1) and the 5th lobule of the cerebellar vermis (5Cb) during unconsciousness induced by sevoflurane and gaining consciousness after sevoflurane exposure. Cerebellar Purkinje cells were optogenetically manipulated to investigate their influence on consciousness transitions during and after sevoflurane exposure.
Results: Activation of 5Cb Purkinje cells increased the Ca2+ flux in the M1 CaMKIIα+ neurons, but this increment was significantly reduced by inactivation of posterior and parafascicular thalamic nucleus. The 5Cb and M1 exhibited concerted calcium flux, and glutamate and GABA release during transitions from wakefulness, loss of consciousness, burst suppression to conscious recovery. Ca2+ flux and Glu release in the M1, but not in the 5Cb, showed a strong synchronization with the EEG burst suppression, particularly, in the gamma-band range. In contrast, the Glu, GABA and NE release and Ca2+ oscillations were coherent with the EEG gamma band activity only in the 5Cb during the pre-recovery of consciousness period. The optogenetic activation of Purkinje cells during burst suppression significantly facilitated emergence from anesthesia while the optogenetic inhibition prolonged the time to gaining consciousness.
Conclusions: Our data indicate that cerebellar neuronal communication integrated with motor cortex through thalamus promotes consciousness recovery from anesthesia which may likely serve as arousal regulation.
Keywords: Consciousness, Cerebellum, Motor cortex, Neurotransmitters, Sevoflurane
 Global reach, higher impact
Global reach, higher impact