13.3
Impact Factor
Theranostics 2024; 14(2):714-737. doi:10.7150/thno.88057 This issue Cite
Research Paper
ALDH1A1 drives prostate cancer metastases and radioresistance by interplay with AR- and RAR-dependent transcription
1. OncoRay-National Center for Radiation Research in Oncology, Faculty of Medicine and University Hospital Carl Gustav Carus, Technische Universität Dresden and Helmholtz-Zentrum Dresden-Rossendorf, Dresden, Germany.
2. Institute of Pathology, University Hospital Schleswig-Holstein, Luebeck, Germany; Pathology, Research Center Borstel, Leibniz Lung Center, Borstel, Germany.
3. Institute of Clinical Chemistry and Laboratory Medicine, Technische Universität Dresden, Dresden, Germany.
4. Technische Universität Dresden, CRTD - Center for Regenerative Therapies TU Dresden and Center for Healthy Aging, Faculty of Medicine and University Hospital Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany.
5. Institute of Anatomy and Experimental Morphology, Center for Experimental Medicine, University Cancer Center Hamburg, University Medical Center Hamburg-Eppendorf, Germany.
6. Department of Radiation Biology, Oslo University Hospital, Oslo, Norway.
7. Helmholtz-Zentrum Dresden-Rossendorf, Institute of Radiooncology-OncoRay, Dresden, Germany.
8. German Cancer Consortium (DKTK), partner site Dresden and German Cancer Research Center (DKFZ), Heidelberg, Germany.
9. Department of Urology, University Hospital and Faculty of Medicine, Technische Universität Dresden, Dresden, Germany.
10. Institute of Human Genetics, Jena University Hospital, Friedrich Schiller University, Jena, Germany.
11. Department of Physics, University of Oslo, Oslo, Norway.
12. National Center for Tumor Diseases (NCT), partner site Dresden: German Cancer Research Center (DKFZ), Heidelberg; Faculty of Medicine and University Hospital Carl Gustav Carus, Technische Universität Dresden, and Helmholtz-Zentrum Dresden-Rossendorf (HZDR), Dresden, Germany.
13. Department of Radiotherapy and Radiation Oncology, Faculty of Medicine and University Hospital Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany.
14. Institute of Anatomy I, Cancer Center Central Germany, Jena, University Hospital, Jena, Germany.
* These authors contributed equally.
§ Senior author.
Abstract
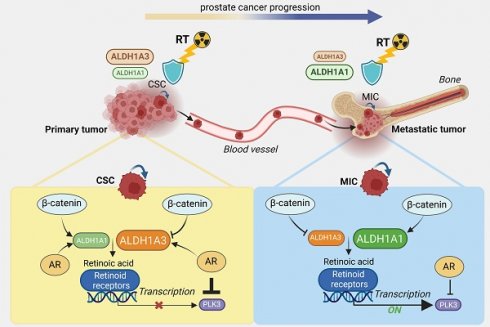
Rationale: Current therapies for metastatic osseous disease frequently fail to provide a durable treatment response. To date, there are only limited therapeutic options for metastatic prostate cancer, the mechanisms that drive the survival of metastasis-initiating cells are poorly characterized, and reliable prognostic markers are missing. A high aldehyde dehydrogenase (ALDH) activity has been long considered a marker of cancer stem cells (CSC). Our study characterized a differential role of ALDH1A1 and ALDH1A3 genes as regulators of prostate cancer progression and metastatic growth.
Methods: By genetic silencing of ALDH1A1 and ALDH1A3 in vitro, in xenografted zebrafish and murine models, and by comparative immunohistochemical analyses of benign, primary tumor, and metastatic specimens from patients with prostate cancer, we demonstrated that ALDH1A1 and ALDH1A3 maintain the CSC phenotype and radioresistance and regulate bone metastasis-initiating cells. We have validated ALDH1A1 and ALDH1A3 as potential biomarkers of clinical outcomes in the independent cohorts of patients with PCa. Furthermore, by RNAseq, chromatin immunoprecipitation (ChIP), and biostatistics analyses, we suggested the molecular mechanisms explaining the role of ALDH1A1 in PCa progression.
Results: We found that aldehyde dehydrogenase protein ALDH1A1 positively regulates tumor cell survival in circulation, extravasation, and metastatic dissemination, whereas ALDH1A3 plays the opposite role. ALDH1A1 and ALDH1A3 are differentially expressed in metastatic tumors of patients with prostate cancer, and their expression levels oppositely correlate with clinical outcomes. Prostate cancer progression is associated with the increasing interplay of ALDH1A1 with androgen receptor (AR) and retinoid receptor (RAR) transcriptional programs. Polo-like kinase 3 (PLK3) was identified as a transcriptional target oppositely regulated by ALDH1A1 and ALDH1A3 genes in RAR and AR-dependent manner. PLK3 contributes to the control of prostate cancer cell proliferation, migration, DNA repair, and radioresistance. ALDH1A1 gain in prostate cancer bone metastases is associated with high PLK3 expression.
Conclusion: This report provides the first evidence that ALDH1A1 and PLK3 could serve as biomarkers to predict metastatic dissemination and radiotherapy resistance in patients with prostate cancer and could be potential therapeutic targets to eliminate metastasis-initiating and radioresistant tumor cell populations.
Keywords: prostate cancer, bone metastases, cancer stem cells, aldehyde dehydrogenase, RARA, androgen receptor, retinoic acid
 Global reach, higher impact
Global reach, higher impact