13.3
Impact Factor
Theranostics 2024; 14(12):4806-4821. doi:10.7150/thno.98476 This issue Cite
Review
The principles and promising future of sonogenetics for precision medicine
1. Department of Ultrasound Medicine, Tangdu Hospital, Fourth Military Medical University, Shaanxi 710038, China.
2. State Key Laboratory of Holistic Integrative Management of Gastrointestinal Cancers, Department of Biochemistry and Molecular Biology, Fourth Military Medical University, Xi'an, Shaanxi 710032, China.
Received 2024-5-15; Accepted 2024-7-29; Published 2024-8-12
Abstract
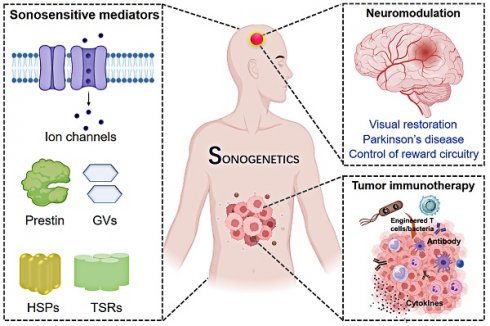
Sonogenetics is an emerging medical technology that uses acoustic waves to control cells through sonosensitive mediators (SSMs) that are genetically encoded, thus remotely and non-invasively modulating specific molecular events and/or biomolecular functions. Sonogenetics has opened new opportunities for targeted spatiotemporal manipulation in the field of gene and cell-based therapies due to its inherent advantages, such as its noninvasive nature, high level of safety, and deep tissue penetration. Sonogenetics holds impressive potential in a wide range of applications, from tumor immunotherapy and mitigation of Parkinsonian symptoms to the modulation of neural reward pathway, and restoration of vision. This review provides a detailed overview of the mechanisms and classifications of established sonogenetics systems and summarizes their applications in disease treatment and management. The review concludes by highlighting the challenges that hinder the further progress of sonogenetics, paving the way for future advances.
Keywords: sonogenetics, ultrasound, biomolecular function, precision medicine, control, sonosensitive mediators.
1. Introduction
Recent advancements in control systems that enable direct manipulation of biomolecular functions through external stimuli show increasing promise for biological research and therapeutic applications [1-3]. Various techniques, including chemical induction [4], optogenetics [5], radiofrequency radiation [6], and magnetogenetics [7], have been developed for an effective management of biomolecules. However, each method presents a unique set of challenges. Chemical inducers often lack precision in timing and location, leading to potential side effects [4]. Optogenetics has limitations related to tissue penetration and typically requires the implantation of optical fibers for targeted applications [5]. Radiofrequency radiation introduces ionizing effects, while magnetogenetics often relies on magnetic nanoparticles to transform magnetic fields into cellular signals, which may compromise specificity. Both radiofrequency and magnetic fields face general challenges related to specificity [6,7]. Therefore, the development of a regulatory tool that is cell-type-selective, non-invasive, and capable of precise, tunable, and high-resolution control of biomolecular functions in vivo is of the utmost importance due to the above limitations.
Sonogenetics is a new ultrasound (US) approach that activates genetically encoded sonosensitive mediators (SSMs) in specific cell types, enabling the precise control of biomolecular functions with high spatiotemporal resolution [8]. Thanks to the advantages of US, sonogenetics possesses a deeper penetration depth than visible light and a higher spatiotemporal precision than magnetic fields, thus overcoming the above limitations of optogenetics and magnetogenetics [9-11]. Sonogenetics operates through two primary mechanisms: thermal [12] and mechanical [13]. The mechanical aspect of sonogenetics exploits US-induced phenomena like streaming [14], cavitation [15], and acoustic radiation forces (ARF) [16] to activate SSMs, facilitating the remote manipulation of biomolecular functions. These mediators include mechanosensitive ion channels such as the two-pore potassium (K2P) family [17], the large conductance mechanosensitive (MscL) family [18,19], the Piezo family [20,21], transient receptor potential (TRP) channels [11], Prestin variant (N7T, N308S) [22], and gas vesicles (GVs) [23]. The thermal effect of US, which induces hyperthermia typically ranging from 37 to 45 °C, modulates heat-sensitive transcription factors, genetic circuits, and thermosensitive transient receptor potential (TRP) cationic channels [12,24-27]. Control is exerted through temperature-sensitive repressors (TSRs), such as TlpA [24] and TcI [25], or promoters, like heat shock promoters (HSPs) [26] and leftward and rightward promoters (PL-PR) [27]. Sonogenetics is a promising approach for the precise regulation of biomolecular functions by integrating both thermal and mechanical gene control elements.
To date, sonogenetics has been successfully applied in various fields. This innovative technology provides a powerful, non-invasive means to manipulate both local and global neuronal functions, enabling the precise modulation of neurons on different brain regions with both high spatial and temporal resolution [28]. Preclinical trials demonstrated the enhancement of specific behavioral outcomes like visual restoration [29], alleviation of Parkinsonian symptoms [30], and modulation of reward circuitry in the ventral tegmental area [31]. Sonogenetics is used in tumor therapy to engineer thermal SSMs and US-controllable target cells for the targeted destruction of tumor cells through inducible therapeutic agent expression [32]. Temperature-based transcription factors and genetic circuits are also engineered to convert the heat generated by US into genetic regulation, enabling the precise control over the expression of therapeutic agents [33]. Furthermore, microbubbles (MBs) and GVs (unique air-filled protein nanostructures) can be used as sono-adjuvants to activate mammalian cells overexpressing SSMs through low-energy US [3]. These systems sensitize the target cells and are widely used in several biomedical applications, enhancing the efficacy and scope of sonogenetics treatments.
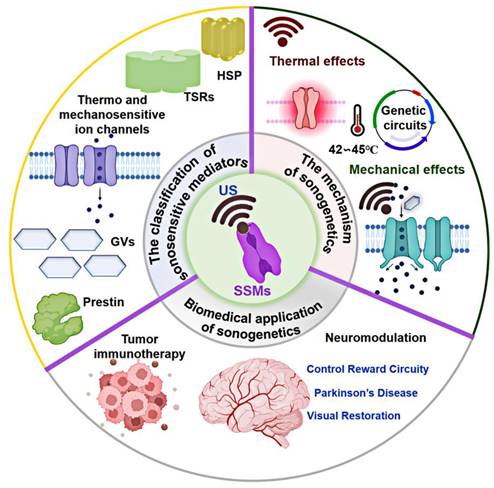
This article offers a thorough and systematic review of sonogenetics covering its classification, generation, mechanisms, and ongoing challenges (as illustrated in Figure 1). First, it investigates the fundamental principles of US and explores their synergy in regulating cellular functions through sonogenetics. Next, it provides an overview of state-of-the-art biomedical applications of sonogenetics and discusses key considerations for a successful clinical application. By providing this background, this review aims to encourage innovative scientific strategies based on sonogenetics capable of addressing a broad spectrum of diseases.
Schematics design of the classification, generation, mechanism, and biomedical application of sonogenetics. Created with BioRender.com.
2. Historical perspective and mechanistic basis of sonogenetics
2.1 History of sonogenetics
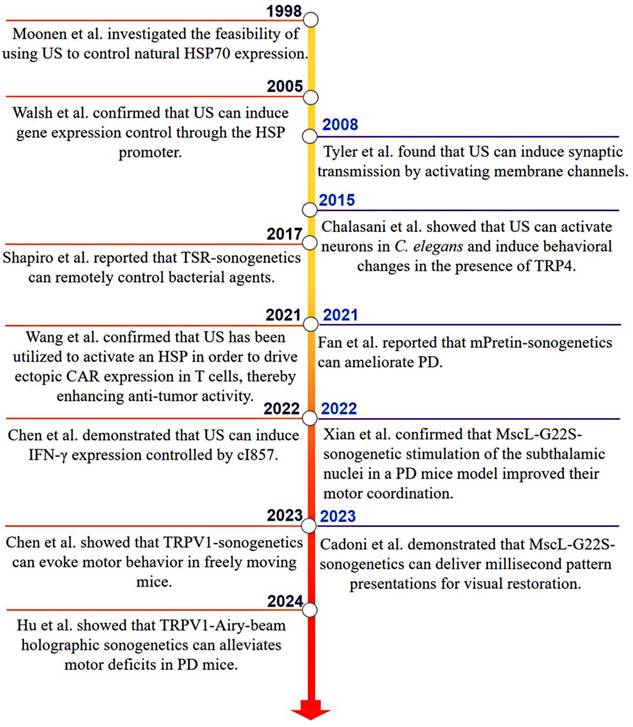
The initial exploration in the regulation of HSP70 through the thermal effects of US dates to 1998, with studies focusing on spatially controlling natural HSP70 expression in vivo [34]. Based on this assumption, subsequent research demonstrated that coupling the HSP promoter with US induces the expression of luciferase genes, marking a significant advance. This significant development paved the way for using HSP promoters in conjunction with US to selectively express therapeutic genes in specific cell populations [35]. In 2008, a separate research group reported that the mechanical effect of US enhances synaptic transmission by activating membrane channels [36].
Although these early studies suggested the potential of sonogenetics, a breakthrough occurred in 2015 when the Chalasani's team successfully activated neurons overexpressing the mechanosensitive ion channel TRP-4 using US. They demonstrated that US modifies the behavior of Caenorhabditis elegans worms in the presence of MBs, and coined the term “sonogenetics” to describe this innovative method [11]. This pivotal study introduced sonogenetics to the wider scientific community, sparking a flurry of research activities. Subsequent proof-of-concept studies have explored a variety of SSMs for US activation, as shown in Figure 2.
Key milestones in the development and application of sonogenetics have been achieved since the previous century, particularly in its biomedical applications. The term “sonogenetics” was introduced around 2015, involving the use of the mechanosensitive ion channel TRP-4 to alter the behavior of Caenorhabditis elegans worms in response to US [11].
2.2 Mechanisms of sonogenetics
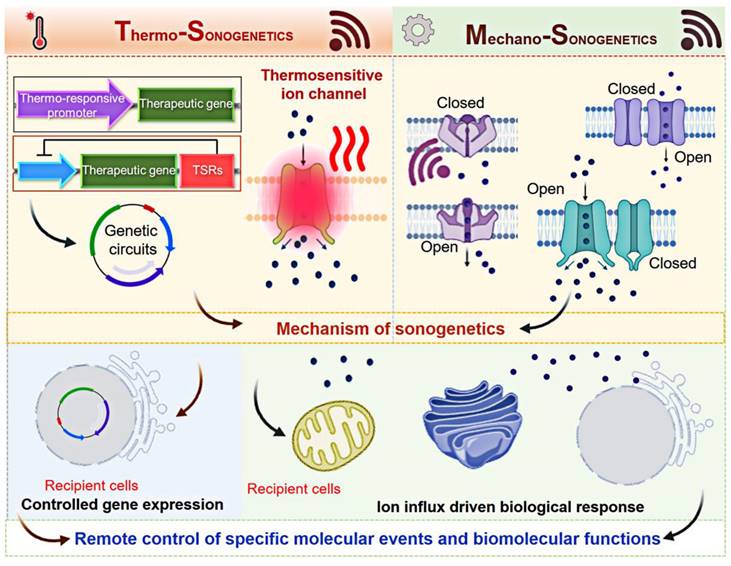
Sonogenetics uses both genetic engineering and US technology, representing a new avenue to control biomolecular function at the molecular level (Figure 3). The mechanism of sonogenetics involves two components: US waves and SSMs.
2.2.1 Ultrasound waves
US is a type of mechanical wave capable of penetrating biological tissues across a range of frequencies, from sub-MHz to several MHz [37]. Focused US (FUS) is a type of US that is spatially focused [38]. Traditionally, US has been used for clinical imaging purposes due to its non-invasive nature and exceptional spatiotemporal resolution [39]. Over the years, US has demonstrated a spectrum of biological effects influenced by parameters such as frequency, power, and duration that are typically classified as “thermal” or “mechanical” [40,41].
As regards thermal effect, US energy is dissipated to thermal energy as the acoustic waves propagate through the tissues and generate friction. FUS leads to localized tissue heating. At high intensities, continuous acoustic waves induce necrosis and thermal stress; in contrast, lower intensities may lead to sub-ablative hyperthermia, characterized by significant but non-lethal heat stress signals [42]. US hyperthermia can be used to turn on thermal bio-switches. Specific examples of thermal bio-switches-mediated sonogenetics are described in this review.
On the one hand, US acoustic waves are propagated through an elastoviscous medium. This propagation actively or passively creates an acoustic pressure gradient based on standing waves, absorption, and tissue acoustic property gradients, and results in the generation of a non-zero net force on the medium, which is known as ARF [43]. As a complement to the thermal effect, ARF applied to sonogenetics is used to induce mechanical signaling. This mechanical force combined with genetically encoded SSMs allows the control of cellular functions by sonogenetics [8]. This review explores specific examples such as the modulation of the excitability of specific neurons and the induction of the release of therapeutic payloads from cells.
A scheme of mechanism of sonogenetics. Thermosensitive ion channels, temperature-sensitive repressors (TSRs), and heat shock proteins (HSPs) are utilized in thermo-sonogenetics, while mechanosensitive ion channels are employed in mechano-sonogenetics. These strategies ultimately enable the control of specific molecular events and biomolecular functions. Created with BioRender.com.
In some cases, US produces cavitation where MBs grow and collapse with compression and rarefaction. MBs undergo symmetrical linear oscillations at low acoustic pressures, while the oscillations become asymmetric under increasing acoustic pressures, promoting the expansion phase [44]. The shear stress from oscillating MBs generates fluid micro-streaming-induced membrane tension variations that activate mechanosensitive ion channels [45]. The MBs-US-induced changes in membrane permeability increase ion flow and lead to changes in membrane potential for control of cellular functions [46].
There are distinct advantages of using thermal and mechanical bio-effects to regulate cellular function, particularly at a level of safety in biological systems and precise spatiotemporal control.
2.2.2 SSMs
Two categories of SSMs function as pivotal links between sonogenetics and cellular function regulation and have been identified across a diverse range of organisms from bacteria to mammals [47,48]. Thermal SSMs are typically inactive under normal physiological conditions presenting intriguing possibilities. For example, the mammalian HSP70 is normally dormant but can be activated by mild hyperthermia (at 43 °C for a brief period) without causing significant tissue damage [49]. Thus, the incorporation of HSP70 into gene circuits allows the precise control of the expression of therapeutic agents in mammalian hosts using sonogenetics. Another significant system involves TSRs, a group of thermal SSMs that regulate gene expression using the phage lambda promoters PL-PR. These systems inhibit gene expression under standard conditions and are only activated when the temperature exceeds 37 °C [50]. The complex interplay of thermal-sensitive mediators in the framework of sonogenetics is further described in Section 3.
In contrast, mechanical SSMs are primarily represented by mechanosensitive ion channels. These channels have unique structures and mechanotransduction mechanisms that respond to US stimulation [51]. It is widely recognized that US deforms cell membranes, indirectly causing conformational changes in channel structures or directly influencing channel components to the transition from a closed to an open state [52-54]. Research has underscored the importance of GVs and the Prestin protein family in eliciting responses to sonogenetics, suggesting that these mediators enhance the activation of US-responsive mechanosensitive ion channels [55,56]. The role of mechanical SSMs in the framework of sonogenetics is described in Section 4.
3. Thermo-sonogenetics
Rapid oscillations in pressure due to US waves, along with the associated cycles of mechanical loading and unloading, generate thermal effects in biological tissues. When applied at high intensity, US rapidly increases the temperature in a focal area from 55 to 80 °C [57,58]. Low-intensity waves induce hyperthermia in targeted regions by carefully adjusting US parameters [59]. Meticulous designs using these specific thermal advantages result in the creation of thermal-sensitive mediators and genetic circuits capable of effectively regulating biomolecular functions (Table 1).
3.1 HSP promoter-mediated sonogenetics
During febrile conditions, HSP promoters are widely used by the body to produce stabilizing proteins [64]. HSP70 promoters are of particular value in gene therapy due to their robust ability to initiate and regulate the transcription of therapeutic genes. The inducible HSP70 promoter amplifies gene expression thousand-fold in response to hyperthermia [65]. A study showed that the HSP70 promoter effectively controls transgene expression under localized hyperthermia conditions both in vitro and in vivo [66]. Furthermore, magnetic resonance imaging (MRI) can be used to monitor US-induced hyperthermia to allow the precise modulation of therapeutic gene expression in a specific temperature range [67]. However, transient US hyperthermia alone is insufficient for a sustained gene activation and cellular function. To overcome this limitation, the Cre-lox gene switch is incorporated into the HSP inducible system. The transient expression of Cre induced by US hyperthermia leads to the excision of the STOP cassette, enabling the switch from ZsGreen expression to the continuous production of the therapeutic agent [33]. Traditional US hyperthermia inducible systems require the cloning of each therapeutic gene under the HSP promoter, which is a challenge in the control of the expression of multiple genes. To address this issue, a more flexible and efficient system has been developed to simultaneously modulate multiple therapeutic genes. This system uses US hyperthermia to control gene expression through an enhanced CRISPR-Cas system. Here, the Cas protein and its effector are regulated by a truncated HSP70 promoter that quickly responds to hyperthermia. A tailored guide RNA array is then used to specifically target one or more therapeutic genes, ultimately facilitating multiplexed activation [10]. This breakthrough highlights the potential of sonogenetics as an innovative approach for enhancing disease treatment by precisely managing molecular events and cellular functions.
Summary of thermal effect-based sonogenetics and their recent applications.
| Mediators | US equipment | Operational methods | US parameters | Application | Tempera-ture | Ref. |
|---|---|---|---|---|---|---|
| TRPV1 | The US equipment was customized by Xinxiang Chiyu Ultrasonic Equipment Co. LTD and consists of a signal generator and a power amplifier. | US-stimulated M1 region of the mouse brain under anesthesia using a collimator. | 1.8 MHz, Stimulation 2 s and stop for 2 s, Duration: 400 s, Power: 6W. | Induction of calcium influx, Neuromodulation in the M1 region of mouse brain. | 42 °C | [60] |
| A specialized wearable FUS device was designed, featuring an FUS transducer and a base plate | Attachment of FUS transducer base plate to the mouse skull. | 1.5 MHz, 40% DC, PRF 10Hz, Duration: 15 s 0.7 and 1.1 MPa. | Neuromodulation of motor cortex in freely moving mice. | 0.7 MPa: 38.57 ± 0.31 °C 1.1 MPa: 39.69 ± 0.59 °C | [61] | |
| HSP | The US field is generated using an unfocused air-backed transrectal transducer, with power delivery managed by a multichannel RF driving system. | A transrectal US transducer surrounded by a water-filled latex membrane is placed in the animal rectum. | 1.5 MHz, Power levels reaching as high as 70 W. | Control of exogenous luciferase expression in canine prostate. | 42 °C | [35] |
| The FUS system consists of a 1.5 MHz 8-element annular array transducer, a 16-channel broadband radiofrequency generator, an X-Y positioning stage, and a degassing and water circulation system. | The FUS transducer is placed directly above the targeted region on the mouse's hindlimb. Thin layers of SCAN ultrasound gel are applied at both the skin-transducer and skin-bed interfaces. | Three pulses of 5 min heating by MRI-guided FUS. | Regulation of CAR-T cell activity in tumors using a Cre-lox gene switch. | 43 °C | [33] | |
| The therapeutic array, operating at 1.5 MHz and comprising 128 elements, was sourced from Imasonic. An imaging array is employed for image guidance and treatment planning. | The needle thermocouple is inserted subcutaneously between the tumor and the body wall. After placement, the animal is positioned laterally with the tumor oriented towards the therapeutic array. | 1.5 MHz 128-element, 0-80% DC, Duration: 15 min, Maximum intensity Ispta of 20 W/cm2. | Multiplexed genome regulation in Jurkat T cells and primary T cells. | 43 °C | [10] | |
| TSR-TlpA36 | The FUS procedure utilizes a 16-channel ultrasound generator, a motorized MRI-compatible transducer positioning system, and an annular array transducer operating at 1.5 MHz. | FUS is used to activate subcutaneously injected bacterial agents at a specific anatomical site. | 1.5 MHz, 41 °C for 45 min to 1 h | Activated engineered E. coli expressing GFP at a specific anatomical site. | 41 °C | [24] |
| TSR-TcI42 | A closed-loop thermal control system was developed to maintain temperature by adjusting the intensity of the FUS. A Velmex BiSlide motorized positioning system is utilized to submerge and position the 0.67 MHz FUS transducer. A signal generator produces the thermal ultrasound signal, which is subsequently amplified and transmitted to the ultrasound transducer. | Mice were anesthetized and secured in a vertical nose-up position to an acrylic arm connected to a manual 3D positioning system, facilitating three-dimensional translation of the mouse within the water bath. The target flank was then activated using the FUS system. | 0.67 MHz, 50% DC, 0.6 MPa, A total of 1 h at 43 °C with 5 min pulse duration. | Activated engineered E. coli Nissle 1917 for the sustained release of aPD-L1 and aATLA-4. | 42 °C | [62] |
| TSR-cI857 | No specific description. | The ON/OFF US irradiation procedure was adjusted to keep the temperature constant, and the mice were irradiated with the ultrasound for 30 min. | 0.96 MHz, 150 ms pulse period, 100 ms pulse width, 3s ON and 5s OFF, 3.52 MPa. | Activated engineered E. coli MG1655 expressing IFN-γ for tumor immunotherapy. | 45 °C | [63] |
3.2 Sonogenetics mediated by TRP cationic channels
Thermosensitive ion channels provide a unique method for manipulating biomolecular functions using US hyperthermia by focusing specifically on the thermosensitive TRP family of channels [68]. A study from the 1990s demonstrated that temperatures above 42 °C activate the TRP vanilloid 1 (TRPV1) ion channel, thereby influencing neuronal synaptic activity [69]. Notably, this activation threshold is only slightly higher than the normal body temperature of most mammals. TRPV1 is thus characterized by inactivity at normal body temperature and activation at approximately 42 °C [70]. In neurons that express TRPV1, the associated peak temperatures in the motor cortex of 38.5-39.7 °C also evoke activation [61]. TRPV1 triggers a significant activation even at low expression, minimizing the risk of toxicity that comes with introducing foreign proteins [70]. TRPV1 has been employed in genetics-based neuromodulation due to its unique properties. For example, TRPV1-based sonogenetics has been used to stimulate motor behavior by targeting the motor cortex in more superficial brain regions under US irradiation with appropriate parameters. Furthermore, this TRPV1 channel has also been used in recent research to control motor behavior in freely moving mice by targeting the striatum in deep brain areas [60,61].
3.3 TSR-mediated sonogenetics
At present, US-TSRs are primarily derived from transcriptional repressors found in bacteria and phages, particularly TlpA and TcI [24]. TlpA is a self-regulator from the virulence plasmid of Salmonella typhimurium that features a C-terminal helix-helix domain that undergoes temperature-dependent unfolding between 37 °C and 45 °C. Its N-terminal DNA-binding domain remains in a low-temperature dimer state, inhibiting the transcription from 52-bp TlpA-operated promoters [71,72]. Shapiro et al. reported the use of the engineered variant TlpA36 for the spatiotemporal control of microbial therapeutics through US. In this study, Escherichia coli expressing GFP under TlpA36 regulation was subcutaneously injected into the hindlimbs of nude mice, and MRI-guided US was applied at one site to maintain a local temperature of 41 °C for 45 min. This approach resulted in specific GFP expression at the targeted site in vivo, illustrating the potential of sonogenetics to manipulate cellular functions in biomedical applications [24].
Subsequently, the same research group reported another effective repressor capable of activation through sonogenetics. TcI is a temperature-sensitive mutant of the bacteriophage lambda protein “cI” that binds to its cognate operator sites in the tandem pR-pL promoter. Repression by TcI can be controlled by significant temperature shifts, with TcI and TcI42 exhibiting activation temperature thresholds of 38 °C and 42 °C, respectively [62]. Additionally, the cI mutant cI857 can be used for temperature-sensitive transcription activation through sonogenetics. At lower temperatures, the cI857 repressor, which is expressed from the same vector, suppresses the transcription from the tandem pR-pL promoter. However, US-induced hyperthermia (45 °C) rapidly deactivates the cI857 repressor, enabling the transcription of therapeutic genes under the pR-pL promoter [63].
4. Mechano-sonogenetics
When US waves encounter reflectors or scatterers with different acoustic impedances, they create a physical momentum that converts sound waves into mechanical force impacting specific tissues [73]. In the case of cavitation, the shear stress from MB oscillations generates fluid micro-streaming [45]. These mechanical forces exerted by US alter the conformations of mechanosensitive proteins, in turn influencing cellular mechanics. This change triggers the activation of mechanosensitive proteins and induces subsequent signaling pathways [74]. This process underpins the development of various mechanosensitive mediators designed to regulate biomolecular functions through sonogenetics (Table 2).
4.1 K2P ion channel-mediated sonogenetics
K2P channels are widely expressed in the human body and contribute to background potassium conductance in various cell types [79-81]. Structured as dimers, each K2P channel consists of two subunits each containing four transmembrane helices (M1 to M4) and two pore-forming domains (P1 and P2) that interact to form a functional channel. The background conductance provided by K2P channels is essential for maintaining the negative membrane potential in cells, thereby reducing the excitability of excitable cells [82]. K2P channels respond to various stimuli and molecules including internal and external pH, phospholipids, voltage, temperature, mechanical stretching of the membrane, interactions with the cytoskeleton, and intracellular signals. Notably, only the TRAAK and TREK channels in the K2P channel family are known as mechanically sensitive [83].
A study using the Xenopus oocyte system to express TREK-1, TREK-2, and TRAAK reported that the currents through these ion channels increased by an average of 23% following US stimulation. This enhancement suggests that sonogenetics modulates the activity of these ion channels, thereby influencing transmembrane currents [17]. Further studies showed that US activates TRAAK ion channels expressed in the cortical neurons of mice, highlighting the potential of TRAAK channels for targeted neuromodulation in sonogenetics to specifically alter the activity of genetically defined cells [75].
4.2 TRP ion channel-mediated sonogenetics
TRP ion channel proteins, which are characterized by six transmembrane segments, are divided into six subfamilies (TRPC, TRPV, TRPM, TRPA, TRPP, and TRPML) based on amino acid sequence similarities [84]. Known for their calcium permeability and diverse activation properties, these channels integrate various stimuli to modulate downstream cell signaling through ion influx and membrane depolarization. The TRP family features a broad array of gating mechanisms that include constitutively active channels as well as those gated by voltage, mechanical forces, temperature, and ligands [85].
Chalasani et al. reported that TRP-4 ion channels, which are crucial pore-forming subunits in mechanotransduction channels, are effectively activated through sonogenetics; however, this activation requires the presence of MBs, which limits its applicability in mammalian models [11]. Subsequent studies have thus aimed to identify more responsive ion channels that do not depend on MBs. For example, low-intensity, low-frequency US stimulates endogenous TRPA1 ion channels in vitro [76]. Furthermore, TRPC1, TRPP2, and TRPM4 in the motor cortex are activated through sonogenetics [13]. Moreover, in vivo experiments showed that human TRPA1 expressed in the motor cortex is activated through sonogenetics [77].
Summary of mechanical effect-based sonogenetics and their applications.
| Family | Mediators | US equipment | Operational methods | US parameters | Application | Ref. |
|---|---|---|---|---|---|---|
| K2P | TRAAK TREK-1 TREK-2 | A tone burs US wave is generated using an immersion focus ultrasonic transducer, and this transducer is driven using a signal generator connected to a broadband amplifier. | Cells are positioned either on a plastic plate or within an aperture between two thin sheets of borosilicate glass at the focal point of the US focus. | 10 MHz, 0.3-4.9 W/cm2. | TRAAK and TREK-1/2 sonogenetics facilitate the modulation of transmembrane currents. | [17] |
| TRAAK | A US wave is generated using a focused-immersion ultrasonic transducer. A function generator is used to trigger the transducer's US pulses. | Inside-out patches excised from either oocytes or proteoliposomes are quickly transferred to the US chamber. | 5 MHz, 0.2-3.6 W/cm2, Duration: 10 ms. | US activates TRAAK ion channels expressed in the cortical neurons of mice. | [75] | |
| TRP | TRP4 | An arbitrary waveform generator triggers a submersible transducer with a 300-W amplifier. | Anesthetized animals are surrounded by a solution of MBs and stimulated using US peak negative pressures. | 0.69-3 MHz, Duration: 10 ms, 0-0.9MPa. | TRP-4-sonogenetics stimulation in PVD sensory/AIY neurons reverses the behavior of MBs-bound C. elegans. | [11] |
| Mouse TRPA1 | US is generated by two function generators connected in series, with the first function generator triggering the operation of the second. The bursts of pulsed sine waves produced by the function generators are amplified by a linear power amplifier and subsequently delivered to a single-element focused US transducer. | The US transducer is affixed above the mouse head, and the focus of the transducer is positioned to target the area of the motor cortex. | 0.35-0.5 MHz, 50%DC, Duration: 100 ms, PRF 1.16, 1.5 and 2 kHz, >0.01MPa. | TRPA1-sonogenetics stimulation in astrocytes induces glutamate release for tail movement. | [76] | |
| hs TRPA1 | US waves generated from custom-made, single-crystalline 127.68 Y-rotated X-propagating lithium niobate transducers are regulated by a waveform generator, with pressure control achieved through a 300-W amplifier. | hsTRPA1 is expressed in the left motor cortex of Npr3-Cre transgenic mice and controlled by US stimuli. | 7 MHz, Duration: 100 ms, 0.15-2.5 MPa. | hsTRPA1 enables sonogenetic activation of mouse layer V motor cortex neurons in vivo. | [77] | |
| TRPC1, TRPP2, and TRPM4 | The transducer is immersed in degassed water and oriented at a 20° angle relative to normal incidence on the Mylar film using a specialized holder. An Axon Digidata 1550 acquisition system is employed to program and produce a specified number of trigger pulses, which are subsequently transmitted to an arbitrary waveform generator. The output from the waveform generator is then amplified by a linear amplifier and utilized to drive the transducer. | Cultured primary murine cortical neurons are placed on an acoustically transparent mylar film at the top of a water tank, with a FUS transducer submerged in degassed water beneath them. The transducer uniformly delivers ultrasound to the neurons. | 0.3 and 0.67 MHz, PRF 1 and 1.5 kHz, Duration: 500 ms, 0-15W/cm2. | Stimulation of TRPC1, TRPP2, and TRPM4 with US in primary cultured cortical neurons induces calcium influx and lowers the threshold for US activation. | [13] | |
| MscL | Mscl-G22S | US equipment consists of a commercial transducer, two function generators, and a power amplifier to produce burst pulses. | Mice are anesthetized, US gel is applied to the shaved head, and the transducer is placed in contact with the gel. | 0.5 MHz, 40%DC, PRF 1kHz, 0.05-0.5 MPa. | Induction of Ca2+ influx. Activation of neurons in vitro. Evoking electromyogram responses in vivo. | [78] |
| US transducer is driven by a set of function generators and power amplifier generating pulsed US. | A wearable US transducer is mounted on the adaptor attached to the skull, coupled with US gel. | 0.5 MHz, PRF:1KHz, Interval: 3s, 0.04-0.35 MPa. | Control of reward circuitry and improved motor coordination in PD mice. | [31] | ||
| A TiePie Handyscope is used to produce the stimulus waveform, which is then passed through an 80 dB RF power amplifier connected to the transducer. | In vitro: US transducers are coupled with a custom-made coupling cone filled with degassed water and mounted on a motorized stage placed orthogonally above the retina. In vivo: US transducers are coupled to the brain with a custom-made coupling cone filled with degassed water and US gel on a motorized stage. | 50% DC, PRF 1kHz, 0.5 MHz: 0.11 - 0.88 MPa, 2.25 MHz: 0.3 - 1.6 MPa, 15 MHz: 0.2-1.27 MPa. | Stimulation of retinal or cortical neurons with MscL-G22S sonogenetics occurs in milliseconds, with acoustic energy deposition compatible with vision restoration. | [29] | ||
| MscL-I92L | Surface-acoustic-wave-based chips are used to generate US, and the transducers are driven using an arbitrary waveform generator connected to a broadband amplifier. | Cells are transferred to the chamber in the US device. | 29.92 MHz, Duration: 50-400 ms, 0.12-0.45 MPa. | MscL-I92L- sonogenetics stimulation of rat primary hippocampal neurons evokes the spikes. | [18] | |
| Piezo | Piezo1 | The ultrasonic transducer is affixed to a three-dimensional mechanical stage to enable precise control over its positioning. A pulser/receiver and an oscilloscope are employed to align the natural focal point of the ultrasonic transducer with the target cell. | A glass tube is used to contain cells, and the ultrasonic transducer is positioned at a 45° angle for the glass surface for ultrasound stimulation. | 2 MHz, 1%DC, PRF 100Hz, Duration: 10 min, Vpp: 22.12-31.6 V. | Piezo1-sonogenetics stimulation of Jurkat T cells and primary T cells induces anti-CD19 CAR. | [14] |
| Prestin | mPrestin (N7T, N308S) | The US transducer is driven by a function generator and a radio frequency power amplifier to transmit the US pulses. | The mice are immobilized using a stereotactic frame and anesthetized throughout the experiment. US is guided by 25-MHz US imaging and delivered transcranially to the brain of the mice. | 0.5MHz, 10%DC, PRF 10Hz, Duration: 3s, 0.5 MPa | mPrestin-sonogenetics- based neuromodulation can alleviate Parkinson's disease symptoms | [30] |
TRPV1 is notable within the TRP family as an ion channel that is activated through US-induced hyperthermia, as discussed in Section 3.
4.3 MscL ion channel-mediated sonogenetics
MscL, which is found in Escherichia coli, is a pentameric channel composed of five subunits, each with two transmembrane helices (TM1 and TM2) and alkaline phosphatase fusion constructs. The TM1 helices from the five subunits cluster tightly to form the pore region, while the TM2 helices embedded in the lipid membrane sense mechanical forces and regulate MscL opening. The TM1 helix, which is highly conserved across MscL channels, is involved in channel gating. Mutations in this region significantly alter channel behavior, increase the mechanical sensitivity of MscL and enable the opening with a minimal membrane tension. Activated MscL channels demonstrate high electrical conductivity (3 nS), thereby facilitating the passage of ions, water, and small proteins [86,87].
Structural modifications to the TM1 and TM2 helices in MscL over time result in a variety of mutants with different levels of mechanical sensitivity [88]. Unlike mechanosensitive ion channels in the K2P and TRP families, prolonged stimulation does not result in inactivation of MscL, making it suitable in the regulation of biomolecular functions through sonogenetics [83]. Heterologous expression of MscL-G22S is mechanically sensitive and is activated by tethered MBs in retinal pigment epithelial (RPE) cells [89]. Cells expressing MscL-G22S respond to sonogenetics stimulation both in vitro and in vivo, leading to Ca2+ influx and neuronal activation in areas expressing MscL [78]. Similar to MscL-G22S, another mutant MscL-I92L also shows increased mechanosensitivity compared to the wild-type, making it an effective mediator of US-activated responses in primary cultured neurons [18].
Nevertheless, there are remaining issues on the large opening of MscL ion channels, potential interactions with other ion channels, and the tendency to aggregate into clusters [90, 91]. Extensive opening allows the passage of ions as well as water and certain proteins, which reduces the mechanosensitivity of MscL when it forms clusters.
4.4 Piezo ion channel-mediated sonogenetics
Mechanosensitive Piezo ion channels are large transmembrane proteins including more than 2,500 amino acids characterized by a unique 38-transmembrane (TM) helix topology that forms a propeller-shaped trimeric structure. This structure features a central ion-conducting pore surrounded by three peripheral mechanosensing blades [92,93]. The distinctively curved TM region of these blades creates a nano-bowl configuration that results in a substantial expansion of the in-plane membrane area, endowing the Piezo channels with exceptional mechanical sensitivity [94]. A previous study showed that Piezo1 forms mechanically activated, non-selective cationic channels with a slight preference for Ca2+ over monovalent sodium ions (Na+) [92]. Heterologous expression of Piezo1 ion channels is activated through sonogenetics in non-neuronal cells [14,95]. For example, sonogenetics activates the heterologous expressed Piezo1 in T-cells, leading to calcium influx and subsequent expression of target genes [14]. Furthermore, Shen et al. developed a Piezo1-targeted microbubble (PTMB) that binds to the extracellular domains of the Piezo1 channel in N2A cells and cultures of primary neurons. The use of these PTMBs facilitates the stimulation effect of US on neuromodulation [96]. However, such activation typically requires high-frequency US or MBs.
4.5 Prestin family-mediated sonogenetics
It is well established that various species are able to detect US and echolocate within their environments. Prestin, an auditory-sensing protein found exclusively in outer cochlear hair cells, has been the subject of extensive in vitro research. Changes in transmembrane voltage induce conformational changes in Prestin, resulting in cellular contraction and elongation, while Prestin deficiency is associated with hearing loss in both humans and mice [97-99]. Since Prestin is a vital component for high-frequency hearing in mammals, it may also be capable of sensing US-induced voltage fluctuations across membranes and converting them into mechanical actions through electrical mobility.
The Prestin mutants (N7T, N308S) demonstrate increased sensitivity to US in vitro, and subsequent studies confirmed their activation by US in vivo [30]. Although a study supports the role of Prestin (SLC26a5) in electromotility and cochlear amplification when activated by US [100], the use of Prestin in sonogenetics requires careful consideration, especially regarding its role in mediating intracellular electromotility.
4.6 GVs as sono-adjuvants
GVs are naturally occurring, nano-sized, gas-filled protein structures in cyanobacteria and archaea that demonstrate a significant potential for US applications. Like MBs, GVs feature a gas core encased by a protein shell, enabling them to mechanically perturb the surrounding tissues through oscillations and/or ARF within the acoustic field [101,102]. It has been confirmed that GVs effectively activate neurons when stimulated by US [56,103]. A proposed mechanism for this effect is an amplification of US-activated mechanosensitive ion channels. The introduction of the mechanosensitive ion channel MscL-G22S into neurons enhances calcium influx and stimulates neuronal activity [103]. Despite these promising findings, several challenges hinder the use of GVs for sonogenetics. Firstly, the effectiveness of GVs-based US activation depends on endogenous mechanosensitive ion channels whose functions are not yet fully understood. Secondly, like heat-responsive circuits, genetic expression of GVs in mammalian cells is complicated as multiple genes are required for their assembly [9].
5. Biomedical applications of sonogenetics
Sonogenetics, which integrates SSMs and US, has emerged as a highly precise method for manipulating biomolecular functions in deep tissues. This section explores the biomedical applications of this innovative technique and highlights the latest advancements in the field of sonogenetics (Figure 4).
5.1 Tumor immunotherapy
Tumors exhibit a complex array of biological abilities, including sustained proliferative signaling, evasion of cell death, resistance to growth suppression, metabolic alterations, promotion of angiogenesis, and facilitation of invasion and metastasis. Tumors often recruit various seemingly normal cells to form the tumor microenvironment, which contributes to these hallmark abilities. As a result of these characteristics, traditional tumor treatments are frequently ineffective [104,105].
In recent years, cell therapies have emerged as promising and effective strategies for treating tumors. Within this context, sonogenetics offers a versatile approach to remotely and non-invasively modulate molecular functions, particularly in cell-based cancer immunotherapy. For example, a mechano-sonogenetics system has been used to remotely control cellular molecular functions [14]. This innovative system involves coupling MBs to the surface of certain cells, such as Jurkat T-cell lines and primary T cells overexpressing the mechanically sensitive Piezo1 ion channel. The Piezo1 ion channels are activated upon US stimulation, leading to enhanced Ca2+ and subsequent activation of downstream pathways, including calcineurin activation, dephosphorylation of the nuclear factor of activated T-cells (NFAT) and its translocation into the nucleus. Subsequently, the translocated NFAT binds to the NFAT response element (NFAT RE [106]) to initiate chimeric antigen receptor (CAR) expression. However, it is difficult to deliver MBs to the tumor site because of their limited half-life, resulting in restricted in vivo applications of MB-activated Piezo1 ion channels. Although calcineurin is calcium-sensitive, the downstream dephosphorylation substrate is not exclusive to NFAT, resulting in a challenge for accurately controlling NFAT dephosphorylation after US activation [107].
In a subsequent study, the same research group developed a thermo-sonogenetics system for controlling engineered T cell activation in vivo using clinically available MRI guidance. This system integrates the Cre-lox gene switch into an inducible sonogenetics system under the regulation of an HSP promoter. This system in Jurkat T-cell lines and primary T cells converts transient thermal stimulation into sustained CAR activation upon US stimulation [33]. While the US stimulation and biocompatible HSP promoter induce a transient expression of various synthetic protein regulators, enhancing the safety of gene therapy and mitigating adverse host immune responses, there are two main challenges hindering the clinical application of this system. Firstly, engineered T cells face difficulties homing to tumor tissues following intravenous delivery. Secondly, the ability of T cells to penetrate and function in the immunosuppressive environment of solid tumors, particularly in hypoxic cores is limited [108-110]. Thus, local injections were necessary to achieve effective therapeutic outcomes due to these challenges.
Immune activity in the tumor core sets the stage for a conducive microenvironment that attracts bacteria, facilitating the precise targeting of the tumor core post-systemic administration [111]. Bacteria-mediated cell therapy has emerged as a promising approach to cancer treatment due to the intrinsic ability of bacteria to infiltrate tumors [112]. The incorporation of sonogenetics promoter engineering and intelligent genetic circuits into bacteria offers a powerful means to finely regulate their function and behavior. For example, sonogenetics-activated engineered bacteria have been used to transiently induce US hyperthermia to trigger the sustained release of immune checkpoint inhibitors targeting CTLA-4 and PD-L1. By adapting TSRs TcI42 to the tumor-infiltrating probiotic species E. coli Nissle 1917 and designing PL-PR temperature-regulated gene circuits, it is possible to effectively control the expression of aCTLA-4 and aPD-L1. Fusion of CTLA-4 and PD-L1 antibodies with a PelB secretion tag has been used to enhance extracellular release of these agents upon US activation. In a study of experimental A20 tumor models, US-activated CTLA-4 and PD-L1 antibodies were successfully activated in situ, leading to significant suppression of tumor growth [62].
Further studies support the feasibility of spatiotemporal control over engineered bacterial function and activity through sonogenetics. Another research group [63] developed a system known as US-responsive engineered bacterium (URB) that is capable of regulating interferon-γ expression in response to US hyperthermia. By inserting the interferon-γ gene under the PL and PR promoters into E. coli MG1655, which specifically accumulates in hypoxic and necrotic tumor regions, the researchers precisely activate anti-tumor immune responses upon US irradiation. Sonogenetics enables the controlled production and secretion of IFN-γ in 4T1 tumor models by engineered E. coli MG1655, inducing apoptosis in 4T1 cancer cells, stimulating the polarization of M2-like macrophages towards an M1-like phenotype, and activating T lymphocytes (CD4+ and CD8+ T cells).
A schematic representation of the biomolecular functions regulated by sonogenetics in tumor modulation and neuromodulation. Introducing engineered T cells or bacteria has enabled several immune strategies to activate antitumor activities through sonogenetics [14,33,62,63]. Conversely, by incorporating mechanosensitive ion channels, sonogenetics enhances specific behavioral outcomes, including visual restoration, mitigation of Parkinsonian symptoms, and modulation of reward circuitry [29,31,116,117]. Created with BioRender.com.
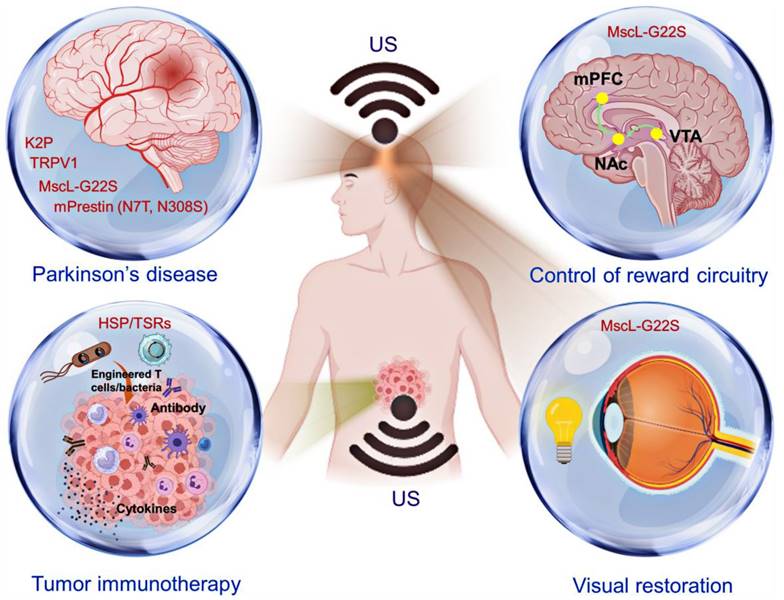
In conclusion, these findings highlight sonogenetics as a valuable tool for precise spatiotemporal control of biomolecular functions in tumor therapy.
5.2 Control of reward circuitry
Dopamine neurons in the ventral tegmental area (VTA) are crucial in the regulation of both adaptive and pathological brain functions associated with reward and motivation. As the principal source of dopamine for critical regions such as the medial prefrontal cortex (mPFC) and nucleus accumbens (NAc), these neurons significantly influence reward circuitry dynamics [113,114]. Sonogenetics stimulation of MscL-G22S in the VTA effectively activates the mesolimbic pathway, leading to dopamine release in the NAc and influencing appetite regulation [31]. Real-time place preference assay indicates that mice with MscL-G22S in their VTA dopamine neurons display increasingly aversive responses when FUS pressure is progressively increased from 0.04 MPa to 0.12 MPa. Fiber photometry has been used to pinpoint the reward circuits activated by the VTA. After stimulating the VTA with 0.3 MPa US, a rapid, synchronous increase in DA2m fluorescence was observed in the NAc of mice expressing MscL-G22S. These findings demonstrate that MscL-G22S-sonogenetics selectively induces dopamine secretion in neurons projecting to the NAc in vivo, thereby eliciting appetitive conditioning behaviors.
Such research sheds light on the complex mechanisms used by sonogenetics to modulate dopamine signaling in the mesolimbic pathway, providing new insights into the potential therapeutic applications of the modulation of reward-related behaviors and appetite regulation
5.3 Parkinson's disease
Parkinson's disease (PD) is the second most common neurodegenerative disorder in humans characterized by symptoms including tremors, bradykinesia, rigidity, and impaired balance [115]. Various studies explored the potential of sonogenetics to alleviate Parkinsonian symptoms. One key in vitro study demonstrated the ability of sonogenetics to protect neuron cells from the toxic effects of 1-Methyl-4-phenylpyridinium (MPP+), a dopaminergic neuronal toxin. This protection was mediated by the activation of K2P ion channels, which trigger downstream pathways involving the phosphoinositide 3-kinase (PI3K)-Akt and ERK1/2 pathways. Although promising, such applications are yet to be applied to animal models of PD [116].
Another study showed that the overexpression of mPrestin (N7T, N308S) in dopaminergic neurons and subjecting them to focused US stimulation results in a significant increase in brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) expression. These changes lead to a notable slowing of dopaminergic neuron degeneration and enhanced dopamine synthesis, resulting in a substantial recovery of motor function in PD model mice [30]. Sonogenetics is also able to modulate neural activity in the subthalamic nucleus (STN) and enhance motor performance in PD mice [31]. By injecting EYFP/MscL-G22S AAVs into the STN region of PD mice, researchers observed that the stimulation of MscL-expressing STN regions leads to an increased c-Fos expression in the STN. PD mice showed significant improvements in motor function following sonogenetics stimulation, as assessed by the rotarod and open-field tests, indicating that sonogenetics targeting MscL-G22S in the STN effectively alleviates motor symptoms.
Although sonogenetics holds promise in alleviating motor deficits in PD mice, existing technologies have limited its potential. Chen et al. recently introduced an Airy-beam holographic sonogenetics (AhSonogenetics) to alleviate motor deficits in mice with PD, addressing the challenge of simultaneously targeting dysfunction in multiple brain regions. By injecting TRPV1 AAVs into the dorsal striatum bilaterally in PD mice, they observed that the stimulation of TRPV1 through AhSonogenetics using the dual-focusing metasurface improved the immobility of PD mice [117].
Overall, these findings highlight the therapeutic potential of sonogenetics for improving motor function and the quality of life in individuals affected by PD.
5.4 Visual restoration
Retinitis pigmentosa is a major cause of blindness, leading to significant sensory impairment. Recent studies explored the potential to restore limited visual perception through retinal stimulation [118]. A groundbreaking 2023 study demonstrated that the combination of MscL-G22S ion channels with unconventional high-frequency US stimulation rapidly activates retinal or cortical neurons with remarkable spatiotemporal precision and acoustic energy deposition conducive to vision restoration [29]. Targeting MscL-G22S in rat retinal ganglion cells (RGCs) leads to a robust and sustained ON spiking responses to sonogenetics stimulation using a 15 MHz US frequency, with most RGCs exhibiting ultra-short latency responses. The activation of MscL-G22S in cortical neurons elicits responses with millisecond latencies and a spatial resolution of at least 400 µm in the x-y plane. Behavioral experiments showed that mice trained to associate visible-light stimulation of one eye with a water reward during water deprivation exhibit an anticipatory lick success rate surpassing that of non-transfected MscL-G22S mice following cortical sonogenetics stimulation, suggesting a level of light perception post-stimulation. Therefore, this new technology characterized by rapid response dynamics, high spatial resolution, and cell-type specificity represents a potential for the advancements in high-resolution visual restoration at the cortical level.
6. Summary and future directions
Sonogenetics is an emerging approach that integrates modular component SSMs with noninvasive US waves technology, providing a new strategy for deep tissue high-resolution and non-invasive therapy. Sonogenetics has promising applications not only in dissecting neural circuits but also in the noninvasive treatment of diseases, such as cancer and neuromodulation. Notably, other than cancer and central nervous system diseases, the range of diseases that might be treated with sonogenetics may be wide, including orthopedic disorders, anaphylaxis, metabolic disease, tissue engineering and regenerative medicine, and others [119-122], for example, this technology may be soon used to activate heart cells and insulin pumps: implanting SSMs in the human heart/pancreas and activating these cells with an external US device could revolutionize pacemakers and diabetes treatment. In the fields of tissue engineering and regenerative medicine, sonogenetics has the potential to modulate tissue maturation spatiotemporally, impact the microenvironment, and alter cellular programming, thereby enabling precise control over the fate of engineered cells and tissues [122]. However, like all emerging technologies, there are ongoing challenges that need to be addressed to treat patients across a broader spectrum of diseases.
6.1 SSMs
SSMs are crucial for ensuring the precise selectivity of sonogenetics. Viral vectors are commonly used to genetically modify specific cells. However, this approach raises safety and cost concerns due to the risk of viral vector leakage into non-targeted cells, potentially causing unintended side effects. In neuromodulation, there have been cases of involuntary activation of the ascending auditory system by US in various species including cats, mice, guinea pigs, and humans [123-126]. Such unintended activation leads to auditory confusion and off-target effects, particularly in neuromodulation applications. Moreover, the distribution of mediators in various brain regions through intracranial injections damages the healthy brain tissue and backflow of viral vectors along the inserted cannula can compromise gene expression.
Ideally, mediators should exhibit high sonosensitivity, enhance safety, and minimize tissue disruption during therapeutic procedures. Although some promising SSMs have been identified, it is crucial to determine which ones show both structural and functional compatibility with sonogenetics responsiveness. Refining the structure and function of these mediators and their mutants may enhance the potential for an efficient remote control of their activity in vivo through sonogenetics. For example, the incorporation of long and highly charged domains enables the efficient transfer of US mechanical force to the protein structure, and successfully realizes the use of different enzymes and switching “on” and “off” the protein activity [127]. In addition, the use of mechanoluminescent nanotransducer to achieve sono-optical energy conversion also enhances sonosensitivity. For example, Wang et al. developed liposome nanotransducers to achieve efficient light emission upon US stimulation. These nanotransducers enable minimal invasive deep brain stimulation through a flexible, mechanoluminescent sono-optogenetic system [128]. Furthermore, exploring non-invasive approaches for mediators, such as using US-induced cavitation effects for blood-brain barrier disruption in specific brain regions and FUS-mediated MB destruction in targeted areas, is also of great promise [129-131]. Future research should combine new minimally invasive drug delivery techniques with SSMs-mediated sonogenetics in small and large animal models to achieve a precise, spatially targeting, and cell type-specific regulation of function and activity.
6.2 US waves
The use of US waves in sonogenetics presents unique challenges, as this requires high spatial resolution and precise control of thermal and mechanical effects. Although high spatial resolution is achieved through high-frequency US (at least 400 µm in the x-y plane at 15 MHz [29]), this comes at the expense of a reduced tissue penetration. For example, high-frequency US (> 10 MHz) is tightly focused to a small volume, but may not effectively penetrate deeper brain structures [77]. Selecting the optimal frequency is thus crucial. Although current approaches are suitable for rodent models, designing a transducer array for nonhuman primates is essential for enhancing both the spatial resolution of acoustic stimuli and the penetration of US waves.
To achieve desired sonogenetics effects while minimizing unintended tissue damage or adverse effects, precise adjustment of US parameters (e.g., frequency, intensity, duration, and waveform) is essential. To date, most studies focusing on the relationship between US parameters and induced biological effects focused their attention on a single type of tissue or cell. Notably, tissues or cells with different acoustic properties may respond differently to sonogenetics stimulation. Therefore, the comprehensive exploration of the key factors influencing the biomedical effects of sonogenetics is crucial to optimize the potency of sonogenetics and guide the development of disease therapy.
The development of user-friendly interfaces for sonogenetics instruments tailored for clinical use is of utmost importance. An ideal sonogenetics device should enable easy adjustment of US parameters, precise acoustic positioning, real-time temperature monitoring, mediator delivery, and seamless integration of clinical diagnostics. Emphasis should be placed on precise parameter control to ensure safety, accounting for factors such as tissue characteristics, cavitation, and acoustic streaming. Thus, maintaining the controlled thermal and mechanical effects of sonogenetics on various tissues and cells is essential to accelerate putative clinical conversion.
Acknowledgements
Funding
This work was financially supported by the Postdoctoral Fellowship Program of China Postdoctoral Science Foundation (No. GZC20233587), the National Natural Science Foundation of China (No. 82272010), and the Key R&D Project in Shaanxi Province (No. 2023-ZDLSF-22).
Ethical approval
This article does not contain any study with human participants or animals performed by any authors.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Matsubara T, Yanagida T, Kawaguchi N, Nakano T, Yoshimoto J, Sezaki M. et al. Remote control of neural function by X-ray-induced scintillation. Nat Commun. 2021;12:4478
2. Zhu I, Liu R, Garcia JM, Hyrenius-Wittsten A, Piraner DI, Alavi J. et al. Modular design of synthetic receptors for programmed gene regulation in cell therapies. Cell. 2022;185:1431-43 e16
3. Hahmann J, Ishaqat A, Lammers T, Herrmann A. Sonogenetics for Monitoring and Modulating Biomolecular Function by Ultrasound. Angew Chem Int Ed Engl. 2024;63:e202317112
4. Stanton BZ, Chory EJ, Crabtree GR. Chemically induced proximity in biology and medicine. Science. 2018;359:eaao5902
5. Mansouri M, Strittmatter T, Fussenegger M. Light-Controlled Mammalian Cells and Their Therapeutic Applications in Synthetic Biology. Adv Sci (Weinh). 2019;6:1800952
6. Stanley SA, Gagner JE, Damanpour S, Yoshida M, Dordick JS, Friedman JM. Radio-wave heating of iron oxide nanoparticles can regulate plasma glucose in mice. Science. 2012;336:604-608
7. Cohen Y, Shoushan SY. Magnetic nanoparticles-based diagnostics and theranostics. Curr Opin Biotechnol. 2013;24:672-681
8. Maresca D, Lakshmanan A, Abedi M, Bar-Zion A, Farhadi A, Lu GJ. et al. Biomolecular Ultrasound and Sonogenetics. Annu Rev Chem Biomol Eng. 2018;9:229-252
9. Liu T, Choi MH, Zhu J, Zhu T, Yang J, Li N. et al. Sonogenetics: Recent advances and future directions. Brain Stimul. 2022;15:1308-1317
10. Liu P, Foiret J, Situ Y, Zhang N, Kare AJ, Wu B. et al. Sonogenetic control of multiplexed genome regulation and base editing. Nat Commun. 2023;14:6575
11. Ibsen S, Tong A, Schutt C, Esener S, Chalasani SH. Sonogenetics is a non-invasive approach to activating neurons in Caenorhabditis elegans. Nat Commun. 2015;6:8264
12. Yang Y, Pacia CP, Ye D, Zhu L, Baek H, Yue Y. et al. Sonothermogenetics for noninvasive and cell-type specific deep brain neuromodulation. Brain Stimul. 2021;14:790-800
13. Yoo S, Mittelstein DR, Hurt RC, Lacroix J, Shapiro MG. Focused ultrasound excites cortical neurons via mechanosensitive calcium accumulation and ion channel amplification. Nat Commun. 2022;13:493
14. Pan Y, Yoon S, Sun J, Huang Z, Lee C, Allen M. et al. Mechanogenetics for the remote and noninvasive control of cancer immunotherapy. Proc Natl Acad Sci U S A. 2018;115:992-997
15. Athanassiadis AG, Ma Z, Moreno-Gomez N, Melde K, Choi E, Goyal R. et al. Ultrasound-Responsive Systems as Components for Smart Materials. Chem Rev. 2022;122:5165-5208
16. Sarvazyan AP, Rudenko OV, Fatemi M. Acoustic Radiation Force: A Review of Four Mechanisms for Biomedical Applications. IEEE Trans Ultrason Ferroelectr Freq Control. 2021;68:3261-3269
17. Kubanek J, Shi J, Marsh J, Chen D, Deng C, Cui J. Ultrasound modulates ion channel currents. Sci Rep. 2016;6:24170
18. Ye J, Tang S, Meng L, Li X, Wen X, Chen S. et al. Ultrasonic Control of Neural Activity through Activation of the Mechanosensitive Channel MscL. Nano Lett. 2018;18:4148-4155
19. Wang T, Wang H, Pang G, He T, Yu P, Cheng G. et al. A Logic AND-Gated Sonogene Nanosystem for Precisely Regulating the Apoptosis of Tumor Cells. ACS Appl Mater Interfaces. 2020;12:56692-56700
20. Liao D, Hsiao MY, Xiang G, Zhong P. Optimal pulse length of insonification for Piezo1 activation and intracellular calcium response. Sci Rep. 2021;11:709
21. Zhu J, Xian Q, Hou X, Wong KF, Zhu T, Chen Z. et al. The mechanosensitive ion channel Piezo1 contributes to ultrasound neuromodulation. Proc Natl Acad Sci U S A. 2023;120:e2300291120
22. Wu CY, Fan CH, Chiu NH, Ho YJ, Lin YC, Yeh CK. Targeted delivery of engineered auditory sensing protein for ultrasound neuromodulation in the brain. Theranostics. 2020;10:3546-3561
23. Wang HC, Phan TN, Kao CL, Yeh CK, Lin YC. Genetically encoded mediators for sonogenetics and their applications in neuromodulation. Front Cell Neurosci. 2023;17:1326279
24. Piraner DI, Abedi MH, Moser BA, Lee-Gosselin A, Shapiro MG. Tunable thermal bioswitches for in vivo control of microbial therapeutics. Nat Chem Biol. 2017;13:75-80
25. Xiong LL, Garrett MA, Buss MT, Kornfield JA, Shapiro MG. Tunable Temperature-Sensitive Transcriptional Activation Based on Lambda Repressor. ACS Synth Biol. 2022;11:2518-2522
26. Kruse DE, Mackanos MA, O'Connell-Rodwell CE, Contag CH, Ferrara KW. Short-duration-focused ultrasound stimulation of Hsp70 expression in vivo. Phys Med Biol. 2008;53:3641-3660
27. Valdez-Cruz NA, Caspeta L, Pérez NO, Ramírez OT, Trujillo-Roldán MA. Production of recombinant proteins in E. coli by the heat inducible expression system based on the phage lambda pL and/or pR promoter. Microb Cell Fact. 2010;9:18
28. Azadeh SS, Lordifard P, Soheilifar MH, Esmaeeli Djavid G, Keshmiri Neghab H. Ultrasound and Sonogenetics: A New Perspective for Controlling Cells with Sound. Iran J Pharm Res. 2021;20:151-160
29. Cadoni S, Demene C, Alcala I, Provansal M, Nguyen D, Nelidova D. et al. Ectopic expression of a mechanosensitive channel confers spatiotemporal resolution to ultrasound stimulations of neurons for visual restoration. Nat Nanotechnol. 2023;18:667-676
30. Fan CH, Wei KC, Chiu NH, Liao EC, Wang HC, Wu RY. et al. Sonogenetic-Based Neuromodulation for the Amelioration of Parkinson's Disease. Nano Lett. 2021;21:5967-5976
31. Xian Q, Qiu Z, Murugappan S, Kala S, Wong KF, Li D. et al. Modulation of deep neural circuits with sonogenetics. Proc Natl Acad Sci U S A. 2023;120:e2220575120
32. Gao T, Niu L, Wu X, Dai D, Zhou Y, Liu M. et al. Sonogenetics-controlled synthetic designer cells for cancer therapy in tumor mouse models. Cell Rep Med. 2024;5:101513
33. Wu Y, Liu Y, Huang Z, Wang X, Jin Z, Li J. et al. Control of the activity of CAR-T cells within tumours via focused ultrasound. Nat Biomed Eng. 2021;5:1336-1347
34. Madio DP, van Gelderen P, DesPres D, Olson AW, de Zwart JA, Fawcett TW. et al. On the feasibility of MRI-guided focused ultrasound for local induction of gene expression. J Magn Reson Imaging. 1998;8:101-104
35. Silcox CE, Smith RC, King R, McDannold N, Bromley P, Walsh K. et al. MRI-guided ultrasonic heating allows spatial control of exogenous luciferase in canine prostate. Ultrasound Med Biol. 2005;31:965-970
36. Tyler WJ, Tufail Y, Finsterwald M, Tauchmann ML, Olson EJ, Majestic C. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS One. 2008;3:e3511
37. ter Haar G. Ultrasound bioeffects and safety. Proc Inst Mech Eng H. 2010;224:363-373
38. Darrow DP. Focused Ultrasound for Neuromodulation. Neurotherapeutics. 2019;16:88-99
39. Carson PL. Ultrasound: Imaging, development, application. Med Phys. 2023;50(Suppl 1):35-39
40. Lalzad A, Wong F, Schneider M. Neonatal Cranial Ultrasound: Are Current Safety Guidelines Appropriate? Ultrasound Med Biol. 2017;43:553-560
41. Sheybani ND, Price RJ. Perspectives on Recent Progress in Focused Ultrasound Immunotherapy. Theranostics. 2019;9:7749-7758
42. Curley CT, Sheybani ND, Bullock TN, Price RJ. Focused Ultrasound Immunotherapy for Central Nervous System Pathologies: Challenges and Opportunities. Theranostics. 2017;7:3608-3623
43. Miller DL, Smith NB, Bailey MR, Czarnota GJ, Hynynen K, Makin IR. et al. Overview of Therapeutic Ultrasound Applications and Safety Considerations. J Ultrasound Med. 2012;31:623-634
44. Sirsi SR, Borden MA. State-of-the-art materials for ultrasound-triggered drug delivery. Adv Drug Deliv Rev. 2014;72:3-14
45. Chen C, Gu Y, Tu J, Guo X, Zhang D. Microbubble oscillating in a microvessel filled with viscous fluid: A finite element modeling study. Ultrasonic. 2016;66:54-64
46. Chen S, Nazeri A, Baek H, Ye D, Yang Y, Yuan J. et al. A review of bioeffects induced by focused ultrasound combined with microbubbles on the neurovascular unit. J Cereb Blood Flow Metab. 2022;42:3-26
47. Chen H, Xie Y, Zhang M, Huang J, Jiang W, Zhang R. et al. An Hsp70 promoter-based mouse for heat shock-induced gene modulation. J Mol Med (Berl). 2024;102:693-707
48. Walton TA, Idigo CA, Herrera N, Rees DC. MscL: channeling membrane tension. Pflugers Arch. 2015;467:15-25
49. Ghadhanfar E, Pavlik A, Turcani M. Effect of thermal preconditioning on Hsp70 expression in the medulla oblongata and on hemodynamics during passive hyperthermia. Brain Res. 2019;1723:146404
50. Xu W, Li S, Bock R, Zhang J. A heat-inducible expression system for external control of gene expression in plastids. Plant Biotechnol J. 2024;22:960-969
51. Chu YC, Lim J, Chien A, Chen CC, Wang JL. Activation of Mechanosensitive Ion Channels by Ultrasound. Ultrasound Med Biol. 2022;48:1981-1994
52. Tyler WJ. The mechanobiology of brain function. Nat Rev Neurosci. 2012;13:867-878
53. Tyler WJ. Noninvasive neuromodulation with ultrasound?. A continuum mechanics hypothesis. Neuroscientist. 2011;17:25-36
54. Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, Yoshihiro A. et al. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 2010;66:681-694
55. Huang YS, Fan CH, Hsu N, Chiu NH, Wu CY, Chang CY. et al. Sonogenetic Modulation of Cellular Activities Using an Engineered Auditory-Sensing Protein. Nano Lett. 2020;20:1089-1100
56. Hou X, Jing J, Jiang Y, Huang X, Xian Q, Lei T. et al. Nanobubble-actuated ultrasound neuromodulation for selectively shaping behavior in mice. Nat Commun. 2024;15:2253
57. Schneider CS, Woodworth GF, Vujaskovic Z, Mishra MV. Radiosensitization of high-grade gliomas through induced hyperthermia: Review of clinical experience and the potential role of MR-guided focused ultrasound. Radiother Oncol. 2020;142:43-51
58. De Maio A, Alfieri G, Mattone M, Ghanouni P, Napoli A. High-Intensity Focused Ultrasound Surgery for Tumor Ablation: A Review of Current Applications. Radiol Imaging Cancer. 2024;6:e230074
59. Zhu L, Altman MB, Laszlo A, Straube W, Zoberi I, Hallahan DE. et al. Ultrasound Hyperthermia Technology for Radiosensitization. Ultrasound Med Biol. 2019;45:1025-1043
60. Wang L, Chang G, Yang M, Xu Z, Wang J, Xu H. et al. The Noninvasive Sonothermogenetics Used for Neuromodulation in M1 Region of Mice Brain by Overexpression of TRPV1. Neuroscience. 2023;527:22-36
61. Xu K, Yang Y, Hu Z, Yue Y, Gong Y, Cui J. et al. TRPV1-mediated sonogenetic neuromodulation of motor cortex in freely moving mice. J Neural Eng. 2023;20:016055
62. Abedi MH, Yao MS, Mittelstein DR, Bar-Zion A, Swift MB, Lee-Gosselin A. et al. Ultrasound-controllable engineered bacteria for cancer immunotherapy. Nat Commun. 2022;13:1585
63. Chen Y, Du M, Yuan Z, Chen Z, Yan F. Spatiotemporal control of engineered bacteria to express interferon-gamma by focused ultrasound for tumor immunotherapy. Nat Commun. 2022;13:4468
64. Voellmy R, Zurcher O, Zurcher M, de Viragh PA, Hall AK, Roberts SM. Targeted heat activation of HSP promoters in the skin of mammalian animals and humans. Cell Stress Chaperones. 2018;23:455-466
65. Moonen CT. Spatio-temporal control of gene expression and cancer treatment using magnetic resonance imaging-guided focused ultrasound. Clin Cancer Res. 2007;13:3482-3489
66. Oda S, Mikami S, Urushihara Y, Murata Y, Kamei Y, Deguchi T. et al. Identification of a functional medaka heat shock promoter and characterization of its ability to induce exogenous gene expression in medaka in vitro and in vivo. Zoolog Sci. 2010;27:410-415
67. Deckers R, Quesson B, Arsaut J, Eimer S, Couillaud F, Moonen CT. Image-guided, noninvasive, spatiotemporal control of gene expression. Proc Natl Acad Sci U S A. 2009;106:1175-1180
68. Kashio M, Tominaga M. TRP channels in thermosensation. Curr Opin Neurobiol. 2022;75:102591
69. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816-824
70. Chen R, Romero G, Christiansen MG, Mohr A, Anikeeva P. Wireless magnetothermal deep brain stimulation. Science. 2015;347:1477-1480
71. Hurme R, Berndt KD, Normark SJ, Rhen M. A proteinaceous gene regulatory thermometer in Salmonella. Cell. 1997;90:55-64
72. Hurme R, Berndt KD, Namork E, Rhen M. DNA binding exerted by a bacterial gene regulator with an extensive coiled-coil domain. J Biol Chem. 1996;271:12626-12631
73. Vasan A, Orosco J, Magaram U, Duque M, Weiss C, Tufail Y. et al. Ultrasound Mediated Cellular Deflection Results in Cellular Depolarization. Adv Sci (Weinh). 2022;9:e2101950
74. Chuang YC, Chen CC. Force From Filaments: The Role of the Cytoskeleton and Extracellular Matrix in the Gating of Mechanosensitive Channels. Front Cell Dev Biol. 2022;10:886048
75. Sorum B, Rietmeijer RA, Gopakumar K, Adesnik H, Brohawn SG. Ultrasound activates mechanosensitive TRAAK K(+) channels through the lipid membrane. Proc Natl Acad Sci U S A. 2021;118:e2006980118
76. Oh SJ, Lee JM, Kim HB, Lee J, Han S, Bae JY. et al. Ultrasonic Neuromodulation via Astrocytic TRPA1. Curr Biol. 2019;29:3386-401 e8
77. Duque M, Lee-Kubli CA, Tufail Y, Magaram U, Patel J, Chakraborty A. et al. Sonogenetic control of mammalian cells using exogenous Transient Receptor Potential A1 channels. Nat Commun. 2022;13:600
78. Qiu Z, Kala S, Guo J, Xian Q, Zhu J, Zhu T. et al. Targeted Neurostimulation in Mouse Brains with Non-invasive Ultrasound. Cell Rep. 2020;32:108033
79. Wiedmann F, Frey N, Schmidt C. Two-Pore-Domain Potassium (K(2P)-) Channels: Cardiac Expression Patterns and Disease-Specific Remodelling Processes. Cells. 2021 10
80. Bista P, Cerina M, Ehling P, Leist M, Pape HC, Meuth SG. et al. The role of two-pore-domain background K(+) (K(2)p) channels in the thalamus. Pflugers Arch. 2015;467:895-905
81. Jin P, Jan LY, Jan YN. Mechanosensitive Ion Channels: Structural Features Relevant to Mechanotransduction Mechanisms. Annu Rev Neurosci. 2020;43:207-229
82. Noel J, Sandoz G, Lesage F. Molecular regulations governing TREK and TRAAK channel functions. Channels (Austin). 2011;5:402-409
83. Brohawn SG. How ion channels sense mechanical force: insights from mechanosensitive K2P channels TRAAK, TREK1, and TREK2. Ann N Y Acad Sci. 2015;1352:20-32
84. Samanta A, Hughes TET, Moiseenkova-Bell VY. Transient Receptor Potential (TRP) Channels. Subcell Biochem. 2018;87:141-165
85. Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619-647
86. Chang G, Spencer RH, Lee AT, Barclay MT, Rees DC. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science. 1998;282:2220-2226
87. Bavi N, Cortes DM, Cox CD, Rohde PR, Liu W, Deitmer JW. et al. The role of MscL amphipathic N terminus indicates a blueprint for bilayer-mediated gating of mechanosensitive channels. Nat Commun. 2016;7:11984
88. Yoshimura K, Usukura J, Sokabe M. Gating-associated conformational changes in the mechanosensitive channel MscL. Proc Natl Acad Sci U S A. 2008;105:4033-4038
89. Heureaux J, Chen D, Murray VL, Deng CX, Liu AP. Activation of a bacterial mechanosensitive channel in mammalian cells by cytoskeletal stress. Cell Mol Bioeng. 2014;7:307-319
90. Sukharev S, Betanzos M, Chiang CS, Guy HR. The gating mechanism of the large mechanosensitive channel MscL. Nature. 2001;409:720-724
91. Grage SL, Keleshian AM, Turdzeladze T, Battle AR, Tay WC, May RP. et al. Bilayer-mediated clustering and functional interaction of MscL channels. Biophys J. 2011;100:1252-1260
92. Jiang Y, Yang X, Jiang J, Xiao B. Structural Designs and Mechanogating Mechanisms of the Mechanosensitive Piezo Channels. Trends Biochem Sci. 2021;46:472-488
93. Zhao Q, Wu K, Geng J, Chi S, Wang Y, Zhi P. et al. Ion Permeation and Mechanotransduction Mechanisms of Mechanosensitive Piezo Channels. Neuron. 2016;89:1248-1263
94. Yang X, Lin C, Chen X, Li S, Li X, Xiao B. Structure deformation and curvature sensing of PIEZO1 in lipid membranes. Nature. 2022;604:377-383
95. Prieto ML, Firouzi K, Khuri-Yakub BT, Maduke M. Activation of Piezo1 but Not Na(V)1.2 Channels by Ultrasound at 43 MHz. Ultrasound Med Biol. 2018;44:1217-1232
96. Shen X, Song Z, Xu E, Zhou J, Yan F. Sensitization of nerve cells to ultrasound stimulation through Piezo1-targeted microbubbles. Ultrason Sonochem. 2021;73:105494
97. Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149-155
98. Dallos P, Fakler B. Prestin, a new type of motor protein. Nat Rev Mol Cell Biol. 2002;3:104-111
99. Rossiter SJ, Zhang S, Liu Y. Prestin and high frequency hearing in mammals. Commun Integr Biol. 2011;4:236-239
100. Santos-Sacchi J, Bai JP, Navaratnam D. Megahertz Sampling of Prestin (SLC26a5) Voltage-Sensor Charge Movements in Outer Hair Cell Membranes Reveals Ultrasonic Activity that May Support Electromotility and Cochlear Amplification. J Neurosci. 2023;43:2460-2468
101. Wu D, Baresch D, Cook C, Ma Z, Duan M, Malounda D. et al. Biomolecular actuators for genetically selective acoustic manipulation of cells. Sci Adv. 2023;9:eadd9186
102. Hill AM, Salmond GPC. Microbial gas vesicles as nanotechnology tools: exploiting intracellular organelles for translational utility in biotechnology, medicine and the environment. Microbiology (Reading). 2020;166:501-509
103. Hou X, Qiu Z, Xian Q, Kala S, Jing J, Wong KF. et al. Precise Ultrasound Neuromodulation in a Deep Brain Region Using Nano Gas Vesicles as Actuators. Adv Sci (Weinh). 2021;8:e2101934
104. Pavlova NN, Zhu J, Thompson CB. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022;34:355-377
105. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674
106. Hogan PG. Calcium-NFAT transcriptional signalling in T cell activation and T cell exhaustion. Cell Calcium. 2017;63:66-69
107. Skupin A, Thurley K. Calcium signaling: from single channels to pathways. Adv Exp Med Biol. 2012;740:531-551
108. Fucà G, Reppel L, Landoni E, Savoldo B, Dotti G. Enhancing chimeric antigen receptor T-cell efficacy in solid tumors. Clin Cancer Res. 2020;26:2444-2451
109. Anderson KG, Stromnes IM, Greenberg PD. Obstacles Posed by the Tumor Microenvironment to T cell Activity: A Case for Synergistic Therapies. Cancer Cell. 2017;31:311-325
110. Maalej KM, Merhi M, Inchakalody VP, Mestiri S, Alam M, Maccalli C. et al. CAR-cell therapy in the era of solid tumor treatment: current challenges and emerging therapeutic advances. Mol Cancer. 2023;22:20
111. Lukasiewicz K, Fol M. Microorganisms in the Treatment of Cancer: Advantages and Limitations. J Immunol Res. 2018;2018:2397808
112. Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc Natl Acad Sci U S A. 2001;98:15155-15160
113. Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM. et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212-217
114. Creed MC, Ntamati NR, Tan KR. VTA GABA neurons modulate specific learning behaviors through the control of dopamine and cholinergic systems. Front Behav Neurosci. 2014;8:8
115. Elsworth JD. Parkinson's disease treatment: past, present, and future. J Neural Transm (Vienna). 2020;127:785-791
116. Zhao L, Feng Y, Shi A, Zhang L, Guo S, Wan M. Neuroprotective Effect of Low-Intensity Pulsed Ultrasound Against MPP(+)-Induced Neurotoxicity in PC12 Cells: Involvement of K2P Channels and Stretch-Activated Ion Channels. Ultrasound Med Biol. 2017;43:1986-1999
117. Hu Z, Yang Y, Yang L, Gong Y, Chukwu C, Ye D. et al. Airy-beam holographic sonogenetics for advancing neuromodulation precision and flexibility. Proc Natl Acad Sci U S A. 2024;121:e2402200121
118. Liu W, Liu S, Li P, Yao K. Retinitis Pigmentosa: Progress in Molecular Pathology and Biotherapeutical Strategies. Int J Mol Sci. 2022;23:4883
119. Yang Y, Yuan J, Field RL, Ye D, Hu Z, Xu K. et al. Induction of a torpor-like hypothermic and hypometabolic state in rodents by ultrasound. Nat Metab. 2023;5:789-803
120. Inoue S, Li C, Hatakeyama J, Jiang H, Kuroki H, Moriyama H. Higher-intensity ultrasound accelerates fracture healing via mechanosensitive ion channel Piezo1. Bone. 2023;177:116916
121. Jiang Z, Chen Z, Xu Y, Li H, Li Y, Peng L. et al. Low-Frequency Ultrasound Sensitive Piezo1 Channels Regulate Keloid-Related Characteristics of Fibroblasts. Adv Sci (Weinh). 2024;11:e2305489
122. Melde K, Athanassiadis AG, Missirlis D, Shi MH, Seneca S, Fischer P. Ultrasound-assisted tissue engineering. Nat Rev Bioeng. 2024;2:486-500
123. Foster KR, Wiederhold ML. Auditory responses in cats produced by pulsed ultrasound. J Acoust Soc Am. 1978;63:1199-1205
124. Sato T, Shapiro MG, Tsao DY. Ultrasonic Neuromodulation Causes Widespread Cortical Activation via an Indirect Auditory Mechanism. Neuron. 2018;98:1031-41 e5
125. Guo H, Hamilton M 2nd, Offutt SJ, Gloeckner CD, Li T, Kim Y. et al. Ultrasound Produces Extensive Brain Activation via a Cochlear Pathway. Neuron. 2018;98:1020-30 e4
126. Braun V, Blackmore J, Cleveland RO, Butler CR. Transcranial ultrasound stimulation in humans is associated with an auditory confound that can be effectively masked. Brain Stimul. 2020;13:1527-1534
127. Zhou Y, Huo S, Loznik M, Göstl R, Boersma AJ, Herrmann A. Controlling Optical and Catalytic Activity of Genetically Engineered Proteins by Ultrasound. Angew Chem Int Ed Engl. 2021;60:1493-1497
128. Wang W, Kevin Tang KW, Pyatnitskiy I, Liu X, Shi X, Huo D. et al. Ultrasound-Induced Cascade Amplification in a Mechanoluminescent Nanotransducer for Enhanced Sono-Optogenetic Deep Brain Stimulation. ACS Nano. 2023;17:24936-24946
129. Blackmore DG, Razansky D, Gotz J. Ultrasound as a versatile tool for short- and long-term improvement and monitoring of brain function. Neuron. 2023;111:1174-1190
130. Wang J, Li Z, Pan M, Fiaz M, Hao Y, Yan Y. et al. Ultrasound-mediated blood-brain barrier opening: An effective drug delivery system for theranostics of brain diseases. Adv Drug Deliv Rev. 2022;190:114539
131. Wu J, Li RK. Ultrasound-targeted microbubble destruction in gene therapy: A new tool to cure human diseases. Genes Dis. 2017;4:64-74
Author contact
![]() Corresponding authors: Pengying Wu, wutdyycom; Lijun Yuan, yuanljedu.cn.
Corresponding authors: Pengying Wu, wutdyycom; Lijun Yuan, yuanljedu.cn.
 Global reach, higher impact
Global reach, higher impact