13.3
Impact Factor
Theranostics 2012; 2(10):976-987. doi:10.7150/thno.5116 This issue Cite
Research Paper
Plasmonic Nanobubbles Rapidly Detect and Destroy Drug-Resistant Tumors
1. Department of Biochemistry and Cell Biology, Rice University, Houston, TX;
2. Department of Cancer Prevention, UT MD Anderson Cancer Center, Houston, TX;
3. Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, TX;
4. Department of Head and Neck Surgery, UT MD Anderson Cancer Center, Houston, TX;
5. Department of Physics and Astronomy, Rice University, Houston, TX, USA.
Abstract
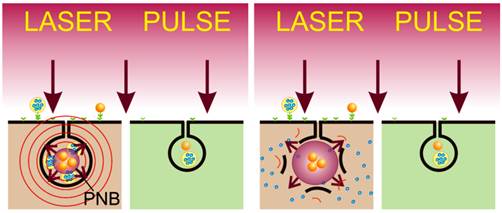
The resistance of residual cancer cells after oncological resection to adjuvant chemoradiotherapies results in both high recurrence rates and high non-specific tissue toxicity, thus preventing the successful treatment of such cancers as head and neck squamous cell carcinoma (HNSCC). The patients' survival rate and quality of life therefore depend upon the efficacy, selectivity and low non-specific toxicity of the adjuvant treatment. We report a novel, theranostic in vivo technology that unites both the acoustic diagnostics and guided intracellular delivery of anti-tumor drug (liposome-encapsulated doxorubicin, Doxil) in one rapid process, namely a pulsed laser-activated plasmonic nanobubble (PNB). HNSCC-bearing mice were treated with gold nanoparticle conjugates, Doxil, and single near-infrared laser pulses of low energy. Tumor-specific clusters of gold nanoparticles (solid gold spheres) converted the optical pulses into localized PNBs. The acoustic signals of the PNB detected the tumor with high specificity and sensitivity. The mechanical impact of the PNB, co-localized with Doxil liposomes, selectively ejected the drug into the cytoplasm of cancer cells. Cancer cell-specific generation of PNBs and their intracellular co-localization with Doxil improved the in vivo therapeutic efficacy from 5-7% for administration of only Doxil or PNBs alone to 90% thus demonstrating the synergistic therapeutic effect of the PNB-based intracellular drug release. This mechanism also reduced the non-specific toxicity of Doxil below a detectable level and the treatment time to less than one minute. Thus PNBs combine highly sensitive diagnosis, overcome drug resistance and minimize non-specific toxicity in a single rapid theranostic procedure for intra-operative treatment.
Keywords: plasmonic nanobubble, cancer cell
 Global reach, higher impact
Global reach, higher impact