13.3
Impact Factor
Theranostics 2015; 5(11):1291-1302. doi:10.7150/thno.12691 This issue Cite
Research Paper
CO2 bubbling-based 'Nanobomb' System for Targetedly Suppressing Panc-1 Pancreatic Tumor via Low Intensity Ultrasound-activated Inertial Cavitation
1. State Key Laboratory of High Performance Ceramics and Superfine Microstructures, Shanghai Institute of Ceramics, Chinese Academy of Sciences, 1295 Ding-Xi Road, Shanghai 200050, P. R. China.
2. Department of Medical Ultrasound, Shanghai Tenth People's Hospital, Tongji University School of Medicine, 301 Yan-chang-zhong Road, Shanghai, 200072, P. R. China.
3. Second Affiliated Hospital of Chongqing Medical University, 76 Linjiang Road, Yuzhong District, Chongqing 400010, P. R. China.
Abstract
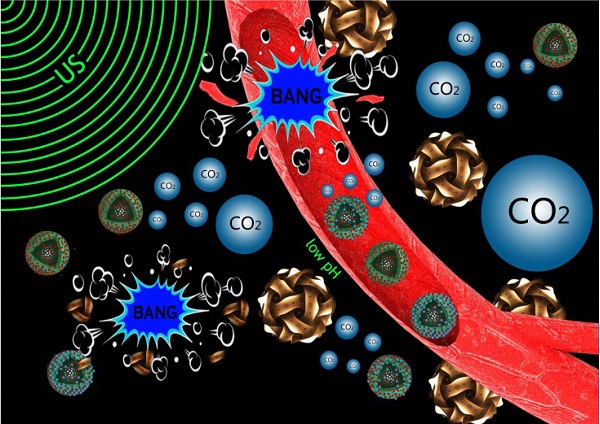
Noninvasive and targeted physical treatment is still desirable especially for those cancerous patients. Herein, we develop a new physical treatment protocol by employing CO2 bubbling-based 'nanobomb' system consisting of low-intensity ultrasound (1.0 W/cm2) and a well-constructed pH/temperature dual-responsive CO2 release system. Depending on the temperature elevation caused by exogenous low-intensity therapeutic ultrasound irradiation and the low pH caused by the endogenous acidic-environment around/within tumor, dual-responsive CO2 release system can quickly release CO2 bubbles, and afterwards, the generated CO2 bubbles waves will timely explode before dissolution due to triggering by therapeutic ultrasound waves. Related bio-effects (e.g., cavitation, mechanical, shock waves, etc) caused by CO2 bubbles' explosion effectively induce instant necrosis of panc-1 cells and blood vessel destruction within panc-1 tumor, and consequently inhibit the growth of panc-1 solid tumor, simultaneously minimizing the side effects to normal organs. This new physiotherapy employing CO2 bubbling-based 'nanobomb' system promises significant potentials in targetedly suppressing tumors, especially for those highly deadly cancers.
Keywords: Nanobomb, inertial cavitation, low intensity ultrasound, panc-1 pancreatic cancer, dual-responsive CO2 release
 Global reach, higher impact
Global reach, higher impact