13.3
Impact Factor
Theranostics 2017; 7(8):2314-2324. doi:10.7150/thno.19710 This issue Cite
Research Paper
Concurrent Chemoradiotherapy with or without Anti-EGFR-Targeted Treatment for Stage II-IVb Nasopharyngeal Carcinoma: Retrospective Analysis with a Large Cohort and Long Follow-up
1. Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, 651 Dongfeng East Road, Guangzhou 510060, P. R. China;
2. Collaborative Innovation Center for Cancer Medicine, State Key Laboratory of Oncology in South China, Sun Yat-sen University Cancer Center
Guangzhou 510060, P. R. China
3. Department of Clinical Research, Sun Yat-sen University Cancer Center, 651 Dongfeng East Road, Guangzhou 510060, P. R. China;
4. Department of Information Technology, Sun Yat-sen University Cancer Center, 651 Dongfeng East Road, Guangzhou 510060, P. R. China;
5. Department of Medical Statistics and Epidemiology, School of Public Health, Sun Yat-sen University, 74 Zhongshan Second Road, Guangzhou 510080, P. R. China;
6. Department of Radiation Oncology, Sun Yat-sen University Cancer Center, 651 Dongfeng East Road, Guangzhou 510060, P. R. China.
* Rui You, Yi-Jun Hua, You-Ping Liu, Qi Yang contributed equally to this study.
Received 2017-2-17; Accepted 2017-4-1; Published 2017-6-1
Abstract
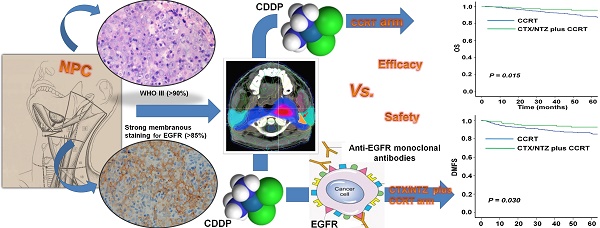
We examined the benefits of the combination of anti-EGFR targeted treatment, cetuximab (CTX) or nimotuzumab (NTZ) and concurrent platinum-based chemoradiotherapy (CCRT) compared with CCRT alone in patients with stage II - IVb nasopharyngeal carcinoma (NPC). A total of 1,628 eligible patients with stage II - IVb NPC, who received CCRT (three cycles of 100 mg/m2 cisplatin every 3 weeks with intensity-modulated radiotherapy) with or without CTX or NTZ between June 2009 and December 2013 were included in the analysis. Using propensity scores to adjust for potential prognostic factors, a well-balanced cohort of 878 patients was created by matching each patient who received CTX or NTZ plus CCRT with no more than four patients who received CCRT alone (1:4). Efficacy and safety were compared between CTX/NTZ plus CCRT and CCRT alone arms. Compared with CCRT alone, treatment with CTX/NTZ plus CCRT was associated with a significantly increased overall survival (3-year OS, 96.6% vs. 92.9%, P = 0.015), improved disease-free survival (3-year DFS, 93.5% vs 86.9%, P = 0.028), and improved distant metastasis-free survival (3-year DMFS, 94.6% vs 89.3%, P = 0.030). Increased rate of CTX related-skin reaction and mucositis was observed in the CTX plus CCRT arm. Multivariate analysis demonstrated the combination of CTX/NTZ was a significant protective factor for OS, DFS, and DMFS in patients treated with CCRT. Our analysis suggests that the addition of CTX/NTZ to CCRT is more effective for maximizing survival in patients with stage II-IVb NPC compared with CCRT alone.
Keywords: nasopharyngeal carcinoma, IMRT, concurrent chemoradiotherapy, nimotuzumab, cetuximab, survival outcome, adverse events.
Introduction
Nasopharyngeal carcinoma (NPC) is highly prevalent in eastern Asia with the highest incidence world-wide reported among the Cantonese population from the province of Guangdong, where rates range from 22.2 to 27.2 per 100000 in males and 9.8 to 11.1 per 100000 in females [1]. Most patients present with stages II-IVb NPC at the time of diagnosis. According to the 2013 National Comprehensive Cancer Network (NCCN) guidelines for head and neck cancer, concurrent chemoradiotherapy (CCRT) is the standard treatment for patients diagnosed with stage II-IVb NPC [2]. Cisplatin (CDDP)-based chemotherapy combined with intensity-modulated radiotherapy (IMRT) has been the most commonly used treatment regimen for these patients in recent years. Given the substantial clinical experience with IMRT and mature data, the available long-term results indicate excellent loco-regional control and improved quality of life [3, 4]. However, this therapy may still fail in 30% of patients. The majority of these treatment failures are due to distant metastasis, especially in patients in a loco-regionally advanced stage [5, 6]. For patients who relapse with distant metastasis, the prognosis is poor with reported median survival ranging from 5 to 11 months [7-9]. Therefore, new systemic strategies are needed for the treatment of NPC.
Epidermal growth factor receptor (EGFR) represents a promising new therapeutic target in cancer. EGFR is highly expressed in most human epithelial carcinomas and has been correlated with a more aggressive phenotype, greater resistance to treatment, and poor prognosis [10, 11]. It has been previously reported that EGFR is expressed in more than 85% of NPC patients. Therefore, anti-EGFR-targeted treatment is considered a potential addition to the standard CCRT regimen for NPC. Cetuximab (CTX) and Nimotuzumab (NTZ), both of which are anti-EGFR monoclonal antibodies, are most frequently utilized in combination treatment of NPC in China. Chan and colleagues first published a multicenter, phase II study in which CTX in combination with carboplatin was demonstrated to have clinical activity and an acceptable safety profile in patients with recurrent or metastatic NPC [12]. They further published another phase II study in which concurrent administration of CTX, CDDP, and IMRT demonstrated a feasible strategy against locoregionally advanced NPC [13]. Furthermore, previous studies reported that NTZ combined with CCRT showed encouraging outcomes in the treatment of locally advanced NPC with no increased toxicity and an improved tolerance in the patients [14, 15]. However, a direct comparison between CCRT alone and CTX/NTZ plus CCRT in NPC was lacking. Therefore, we conceived and initiated a non-profit, retrospective study to directly compare CTX/NTZ plus CCRT and CCRT alone in terms of efficacy and safety in nasopharyngeal carcinoma patients.
Patients and Methods
Patients and study design
An inpatient database at the Sun Yat-sen University Cancer Center (SYSUCC) between January 2009 and December 2013 was used to identify 5,721 patients who were newly diagnosed with NPC. The disease was restaged according to the International Union Against Cancer/American Joint Committee on Cancer (UICC/AJCC) TNM classification (7th edition, 2011) based on clinical and radiography data [16]. The pretreatment evaluation is presented in the Supplementary Material. The inclusion criteria included the following: (a) histologically confirmed NPC; (b) disease classified as stages II-IVb; (c) patient received CCRT; (d) concurrent chemotherapy was cisplatin-based; (e) radiation delivery technique was IMRT; (f) molecularly-targeted drug was CTX or NTZ with at least one cycle administered. The exclusion criteria were as follows: (a) the patient was diagnosed with a previous malignancy or other concomitant malignant disease; (b) the use of adjuvant chemotherapy and induction chemotherapy or additional concurrent systemic therapy other than CDDP, CTX, and NTZ; (c) treatment with weekly cisplatin. From these criteria, 1,628 patients were selected for the matched study (Supplementary Table 1).
We performed an analysis of variance, as well as a χ2 test, on the patients' baseline demographics and clinical characteristics. Variable differences were identified between the 2 groups, including gender, age, the Karnofsky performance status score (KPS), tumor stage (T stage) and node stage (N stage), disease stage, all of which were identified as prognostic factors for survival outcomes in a previous study [17]. Using propensity scores to adjust for these 6 factors, we created a well-balanced cohort by matching each patient who underwent CTX/NTZ plus CCRT with no more than four patients who underwent IMRT plus CDDP within the same year. From this stratification process, we selected a total of 878 patientscomprised of 189 patients in the CTX/NTZ plus CCRT arm and 689 patients in the CCRT arm (Table 1). We first conducted case-matched comparison between the CTX/NTZ plus CCRT and CCRT arms in terms of efficacy and safety in this well-balanced cohort of 878. Subsequently, we conducted multivariate and subgroup analyses based on all 1,628 eligible cases (Figure 1). The clinical research ethics committee of SYSUCC approved this study.
Treatment at referral
Treatment at referral is included in the Supplementary Material.
CONSORT flow diagram
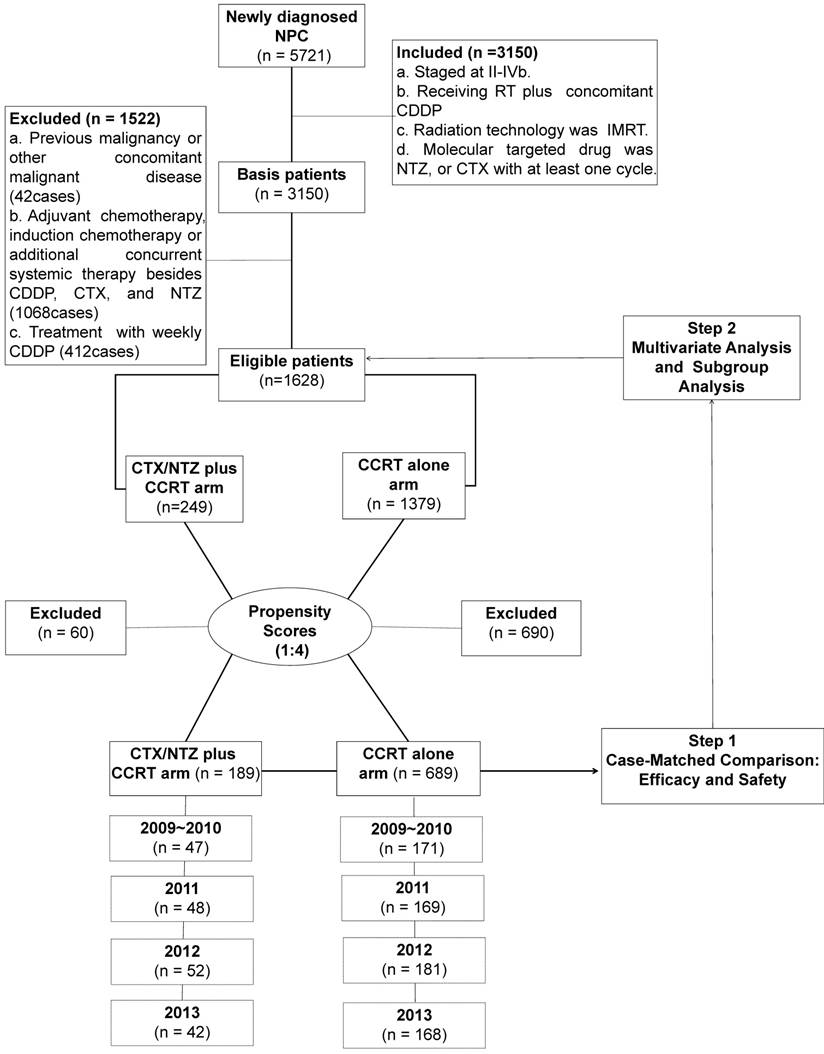
Baseline characteristics of patients in the 878 well-balanced cohort
| Characteristic | CTX/NTZ plus CCRT N = 189 | CCRT N = 689 | P* |
|---|---|---|---|
| Gender | 0.382 | ||
| Female | 39 (20.6%) | 123 (17.9%) | |
| Male | 150 (79.4%) | 566 (82.1%) | |
| Age ——yr | 0.308^ | ||
| Median | 44.7 | 45.6 | |
| Range | 11.8-68.5 | 15.0-74.0 | |
| Karnofsky performance status score | 0.856 | ||
| 90-100 | 173 (91.5%) | 620 (90.0%) | |
| 70-80 | 16 (8.5%) | 69 (10.0%) | |
| T classification | 0.290 | ||
| T1 | 14 (7.4%) | 34 (4.9%) | |
| T2 | 33 (17.5%) | 95 (13.8%) | |
| T3 | 116 (61.4%) | 460 (66.8%) | |
| T4 | 26 (13.8%) | 100 (14.5%) | |
| N classification | 0.933 | ||
| No | 21 (11.1%) | 80 (11.6%) | |
| N1 | 86 (45.5%) | 328 (47.6%) | |
| N2 | 71 (37.6%) | 245 (35.6%) | |
| N3 | 11 (5.8%) | 36 (5.2%) | |
| Disease stage | 0.285 | ||
| II | 23 (12.2%) | 58 (8.4%) | |
| III | 130 (68.8%) | 497 (72.1%) | |
| IV | 36 (19.0%) | 134 (19.4%) |
CCRT = concurrent chemoradiotherapy; NTZ = Nimotuzumab; CTX = Cetuximab; *χ²test or Fisher's exact test. ^ Mann-Whitney U-test.
Follow-up and oncological outcomes
During the course of irradiation, patients were examined weekly. The post-treatment clinical follow-up was generally performed at 3-month intervals for the first 2 years and every 6 months thereafter. The evaluation procedures were similar with those carried out at the pretreatment evaluation. Treatment responses were assessed with nasopharyngeal and neck MRI and flexible nasopharyngoscopy 16 weeks after radiotherapy according to the Response Evaluation Criteria in Solid Tumors (version 1.1). Chemotherapy-related toxic effects were evaluated according to the Common Terminology Criteria for Adverse Events (version 4.0), and radiotherapy-related toxic effects were evaluated according to the Late Radiation Morbidity Scoring Criteria of the Radiation Therapy Oncology Group [18]. Acute toxicities were defined as those occurring either during the course of IMRT or within 90 days of its completion.
Statistical analysis
Statistical analysis is included in the Supplementary Material.
Results
The Treatment Characteristics and Compliance
There was no significant difference in pretreatment imaging methods between the two treatment arms (Supplementary Table 2).
Among the two anti-EGFR monoclonal antibodies used in 189 patients in this study, CTX was more frequently used (102/189, 54.0%) compared with NTZ (87/189, 46.0%). For both NTZ and CTX, the number of utilized cycles was more than one. More patients in the NTZ plus CCRT arm than in the CTX plus CCRT arm completed six to seven cycles of the anti-EGFR monoclonal antibodies (94.3% vs. 85.3%, P = 0.046). No dose reductions were observed in both CTX and NTZ arms (Supplementary Table 3).
All 189 patients in the CTX/NTZ plus CCRT group and all 689 patients in the CCRT group completed IMRT as recommended by the protocol. In both treatment groups, the overall median radiotherapy dose was 70 Gy (IQR 70-70), the overall median dose per fraction was 2.19 Gy (IQR 2.12-2.26), and the overall median duration of radiotherapy was 46 days (IQR 44-49). In concurrent chemotherapy modalities, 178 of 189 patients (94.2%) in the CTX/NTZ plus CCRT group and 655 of 689 patients (95.1%) in the CCRT group completed at least two cycles of CDDP during CCRT, whereas 59 of 189 patients (31.2%) in the CTX/NTZ plus CCRT group and 212 of 689 patients (30.8%) in the CCRT group completed three cycles of CDDP (all P > 0.05). As for the chemotherapy treatment, 123 of 189 patients (75.1%) in the CTX/NTZ plus CCRT group and 473 of 689 patients (72.7%) in the CCRT group received at least 200 mg/m2 (P>0.05) (Supplementary Table 4). The mean dose intensity for concurrent NTZ, CTX was 90.2%, 87.0%, respectively and for concurrent CDDP was 71.3% in the CTX/NTZ plus CCRT arm and 71.7% in the CCRT arm (Supplementary Figure 1).
Efficacy
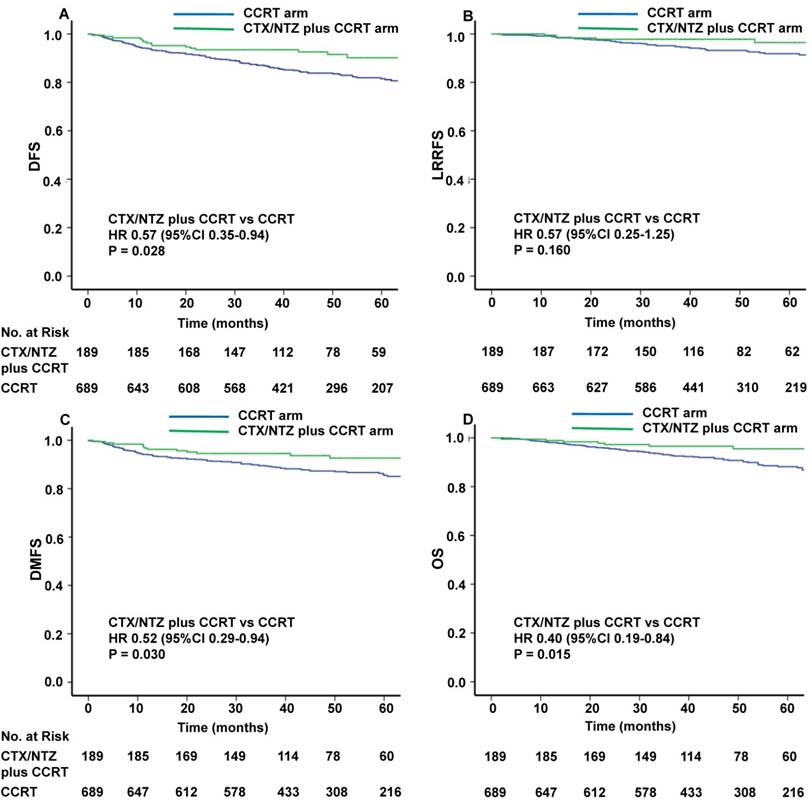
The median time of follow-up was 48.0 months (range, 0-95 months) and 48.9 months (range, 2-93 months) in the CTX/NTZ plus CCRT arm and CCRT arm, respectively. The differences in efficacy between these two groups are presented in Figure 2. The risk of disease progression was lower among the patients treated with CTX/NTZ plus CCRT compared with those treated with CCRT alone (hazard ratio HR for DFS, 0.57; 95% confidence interval CI, 0.35-0.94; P=0.028). The one-, two-, and three-year rates of DFS achieved with CTX/NTZ plus CCRT (96.3%, 93.5%, and 93.5%) were higher than those achieved with CCRT alone (94.0%, 91.0%, and 86.9%). The risk of distant metastasis was lower among the patients treated with CTX/NTZ plus CCRT compared with those treated with CCRT alone (HR 0.52, 95% CI, 0.29-0.94; P=0.030). Similarly, the one-, two-, and three-year rates of DMFS achieved with CTX/NTZ plus CCRT (96.3%, 94.6%, and 94.6%) were higher than those achieved with CCRT alone (94.0%, 91.9%, and 89.3%). A significantly lower risk of death was observed in patients who received CTX/NTZ plus CCRT than in those receiving CCRT alone (HR 0.40, 95% CI 0.19-0.84, P=0.015). The one-, two-, and three-year rates of OS were 98.9%, 97.2%, and 96.6%, respectively, in the CTX/NTZ plus CCRT arm, and 98.1%, 95.5% and 92.9%, respectively, in the CCRT arm. There was no significant difference in the risk of loco-regional relapse in the patients who received CTX/NTZ plus CCRT compared with those who received CCRT alone (HR 0.57, 95% CI 0.25-1.25, P=0.160). The survival rates at one-, two-, three-years achieved with CTX/NTZ plus CCRT (99.5%, 97.8%, and 97.8%) were also similar to those achieved with IMRT plus CDDP (99.0%, 97.0%, and 94.7%).The proportion of patients achieving a complete response 16 weeks after the completion of radiotherapy was high in both groups and did not differ between the groups (Supplementary Table 5).
Kaplan-Meier curves of disease-free survival (A), loco-regional recurrence-free survival (B), distant metastasis-free survival (C), and overall survival (D) with CCRT or CCRT+CTX/NTZ.
Safety
Table 2 displays the toxicity scores of each arm. There was no significant difference in hematological toxicities among CTX plus CCRT arm, NTZ plus CCRT arm, and CCRT arm (P>0.05). The rates of severe hematological toxicities of Grades 3-4 were 19.6% in the CTX plus CCRT arm, 21.8% in the NTZ plus CCRT arm, and 19.4% in the CDDP arm (all P>0.05).
Acute toxicities in NPC patients receiving different treatment regimens
| Acute Toxicity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CTX plus CCRT (N=102) | NTZ plus CCRT (N=87) | CCRT (N=689) | P1 | P2 | P3 | ||||||
| Anemia | ns | ns | ns | ||||||||
| G0-G1 | 78 (76.4%) | 67 (77.0%) | 528 (76.6%) | ||||||||
| G2 | 21 (20.6%) | 16 (18.4%) | 135 (19.6%) | ||||||||
| G3 | 2 (2.0%) | 3 (3.4%) | 19 (2.8%) | ||||||||
| G4 | 1 (1.0%) | 1 (1.1%) | 7 (1.0%) | ||||||||
| Thrombocytopenia | ns | ns | ns | ||||||||
| G0-G1 | 92 (90.2%) | 76 (87.4%) | 605 (87.8%) | ||||||||
| G2 | 8 (7.8%) | 9 (10.3%) | 63 (9.1%) | ||||||||
| G3 | 1 (1.0%) | 2 (2.3%) | 19 (2.8%) | ||||||||
| G4 | 1 (1.0%) | 0 (0.0%) | 2 (0.3%) | ||||||||
| Neutropenia | ns | ns | ns | ||||||||
| G0-G1 | 71 (69.6%) | 63 (72.4%) | 498 (72.3%) | ||||||||
| G2 | 21 (20.6%) | 15 (17.2%) | 125 (18.1%) | ||||||||
| G3 | 10 (9.8%) | 9 (10.3%) | 61 (8.9%) | ||||||||
| G4 | 0 (0.0%) | 0 (0.0%) | 5 (0.7%) | ||||||||
| Leukopenia | ns | ns | ns | ||||||||
| G0-G1 | 52 (51.0%) | 43 (49.4%) | 367 (53.2%) | ||||||||
| G2 | 31(30.4%) | 25 (28.7%) | 189 (27.4%) | ||||||||
| G3 | 19 (18.6%) | 18 (20.7%) | 130 (18.9%) | ||||||||
| G4 | 0 (0.0%) | 1 (1.1%) | 3 (0.4%) | ||||||||
| Hematologic toxicity ≥ G3 | 20 (19.6%) | 19 (21.8%) | 134 (19.4%) | ns | ns | ns | |||||
| Skin reaction | |||||||||||
| G0-G1 | 20 (19.6%) | 57 (65.5%) | 465 (67.5%) | 0.008 | 0.005 | ns | |||||
| G2 | 39 (38.2%) | 25 (28.7%) | 196 (28.4%) | ||||||||
| G3 | 43 (42.2%) | 5 (5.7%) | 28 (4.1%) | ||||||||
| Mucositis | 0.023 | 0.018 | ns | ||||||||
| G0-G1 | 20 (19.6%) | 29 (33.3%) | 177 (25.6%) | ||||||||
| G2 | 28 (27.5%) | 30 (34.5%) | 287 (41.7%) | ||||||||
| G3 | 44 (43.1%) | 25 (28.7%) | 208 (30.2%) | ||||||||
| G4 | 10 (9.8%) | 3 (3.4%) | 17 (2.5%) | ||||||||
| Nausea | ns | ns | ns | ||||||||
| G0-G1 | 35 (34.3%) | 28 (32.2%) | 183 (26.6%) | ||||||||
| G2 | 51 (50.0%) | 48 (55.2%) | 404 (58.6%) | ||||||||
| G3 | 15 (14.7%) | 11 (12.6%) | 90 (13.1%) | ||||||||
| G4 | 1 (1.0%) | 0 (0.0%) | 12 (1.7%) | ||||||||
| Vomiting | ns | ns | ns | ||||||||
| G0-G1 | 78 (76.5%) | 68 (78.2%) | 529 (76.8%) | ||||||||
| G2 | 14 (13.7%) | 8 (9.2%) | 74 (10.7%) | ||||||||
| G3 | 10 (9.8%) | 11 (12.6%) | 83 (12.0%) | ||||||||
| G4 | 0 (0.0%) | 0 (0.0%) | 3 (0.4%) | ||||||||
| Diarrhea | ns | ns | ns | ||||||||
| G0-G1 | 86 (84.3%) | 77 (88.5%) | 628 (91.1%) | ||||||||
| G2 | 12 (11.8%) | 7 (8.0%) | 47 (6.8%) | ||||||||
| G3 | 4 (3.9%) | 3 (3.4%) | 14 (2.0%) | ||||||||
| Hepatoxicity | ns | ns | ns | ||||||||
| G0-G1 | 88 (86.3%) | 75 (86.2%) | 614 (89.1%) | ||||||||
| G2 | 10 (9.8%) | 9 (10.3%) | 57 (8.3%) | ||||||||
| G3 | 4 (3.9%) | 3 (3.4%) | 18 (2.6%) | ||||||||
| Nephrotoxicity | ns | ns | ns | ||||||||
| G0-G1 | 93 (91.2%) | 81 (93.1%) | 630 (91.4%) | ||||||||
| G2 | 6 (5.9%) | 4 (4.6%) | 38 (5.5%) | ||||||||
| G3 | 3 (2.9%) | 2 (2.3%) | 21 (3.0%) | ||||||||
| Weight loss | ns | ns | ns | ||||||||
| G0-G1 | 65 (63.7%) | 61 (70.1%) | 476 (69.1%) | ||||||||
| G2 | 31 (30.4%) | 22 (25.3%) | 192 (27.9%) | ||||||||
| G3 | 6 (5.9%) | 4 (4.6%) | 21 (3.0%) | ||||||||
P1 value was calculated between CTX plus CCRT arm and NTZ plus CCRT arm; P2 value was calculated between CTX plus CCRT arm and CCRT arm; P3 value was calculated between NTZ plus CCRT arm and CCRT arm. ns, non-significant.
No significant differences among the three treatment arms were observed in terms of hepatoxicity, nephrotoxicity, and gastrointestinal reactions including nausea, vomiting, and diarrhea (all P>0.05). A higher frequency of Grades 3 skin reactions was observed in the CTX plus CCRT arm compared with the NTZ plus CCRT arm and CCRT arm (42.2% vs. 5.7%, P=0.008; 42.2% vs. 4.1%, P=0.005). Severe mucositis of Grades 3-4 was more common in the CTX plus CCRT arm compared with the NTZ plus CCRT arm and CCRT arm (52.9% vs. 32.1%, P=0.023; 52.9% vs. 32.7%, P=0.018, respectively), whereas no significant differences in mucositis were observed between the CCRT arm and the NTZ plus CCRT arm. There was no significant difference in weight loss among CTX plus CCRT arm, NTZ plus CCRT arm, and CCRT arm (Table 2).
Multivariate analysis and Subgroup analysis
Univariate and Multivariate analyses of all 1,628 patients further demonstrated that there were statistically significant lower risks of disease progression (HR 0.58, 95% CI 0.36-0.94, P=0.028; HR 0.57, 95% CI 0.35-0.92, P=0.021, for univariate and multivariate analyses, respectively), distant metastasis (HR 0.49, 95% CI 0.28-0.90, P=0.028; HR 0.55, 95% CI 0.31-0.91, P=0.031, respectively), and death (HR 0.42, 95% CI 0.21-0.86, P=0.018; HR 0.40, 95% CI 0.19-0.82, P=0.012, respectively) among patients treated with CTX/NTZ plus CCRT compared with those treated with CCRT alone (Supplementary Table 6 and Table 3).
Multivariate Cox regression analyses further demonstrated that advanced N stage was a significant risk factor for DFS, LRRFS, DMFS, and OS (N3 vs. N0-1, HR 3.96, 95% CI 1.66-9.45, P=0.002; HR 4.76, 95% CI 1.14-19.90, P=0.032; HR 2.89, 95% CI 1.04-8.07, P=0.043; and HR 5.23, 95% CI 1.90-14.40, P=0.001, respectively). In addition, advanced T stage was validated as a significant risk factor for DFS and OS (T4 vs. T1-3, HR 2.80, 95% CI 1.10-7.10, P=0.030; HR 3.32, 95% CI 1.12-9.89, P=0.031) (Table 3).
When analyzing all 1,628 patients, after adjusting for gender, age, KPS, T stage, N stage and disease stage, the interaction analysis showed no significant interaction effect between treatment regimen status (CTC/NTZ plus CCRT vs CCRT) and N stage on DFS, LRRFS, DMFS, and OS (all P > 0.05). Also, there were no interaction effects between treatment regimen status (CTC/NTZ plus CCRT vs CCRT) and T stage on DFS, LRRFS, DMFS, and OS (all P > 0.05) (Table 4).
Discussion
To the best of our knowledge, this study is the first to directly compare CCRT and concomitant CTX/NTZ and CCRT treatments in NPC based on the largest number of patients reported that included 249 patients treated with CTX/NTZ plus CCRT, and 1,379 patients treated with CCRT alone. Our data showed that treatment with CTX/NTZ plus CCRT was associated with significantly improved DFS, DMFS, and OS, but not LRRFS in staged II-IVb NPC.
Multivariate analysis of variables correlated with the treatment regimen status and other prognostic factors
| HR | Cl (95%) | P value | |
|---|---|---|---|
| Disease-free survival | |||
| Treatment regimen status | |||
| CCRT | Reference | ||
| CTX/NTZ plus CCRT | 0.57 | 0.35-0.92 | 0.021 |
| Gender | |||
| Female | Reference | ||
| Male | 1.22 | 0.89-1.67 | 0.221 |
| Age | |||
| <45 | Reference | ||
| ≥45 | 1.15 | 0.88-1.49 | 0.307 |
| Karnofsky performance status score | |||
| 70-80 | Reference | ||
| 90-100 | 1.17 | 0.63-1.65 | 0.497 |
| Tumor stage | |||
| T1-T3 | Reference | ||
| T4 | 2.80 | 1.10-7.10 | 0.030 |
| Node stage | |||
| N0-N1 | Reference | ||
| N2 | 1.46 | 1.11-1.93 | 0.007 |
| N3 | 3.96 | 1.66-9.45 | 0.002 |
| Disease stage | |||
| II-III | Reference | ||
| IV | 0.65 | 0.25-1.72 | 0.386 |
| Loco-regional relapse-free survival | |||
| Treatment regimen status | |||
| CCRT | Reference | ||
| CTX/NTZ plus CCRT | 0.54 | 0.25-1.18 | 0.122 |
| Gender | |||
| Female | Reference | ||
| Male | 1.14 | 0.71-1.84 | 0.591 |
| Age | |||
| <45 | Reference | ||
| ≥45 | 1.04 | 0.70-1.56 | 0.846 |
| Karnofsky performance status score | |||
| 70-80 | Reference | ||
| 90-100 | 0.98 | 0.57-3.56 | 0.658 |
| Tumor stage | |||
| T1-T3 | Reference | ||
| T4 | 4.65 | 0.94-23.00 | 0.060 |
| Node stage | |||
| N0-N1 | Reference | ||
| N2 | 1.23 | 0.80-1.90 | 0.350 |
| N3 | 4.76 | 1.14-19.90 | 0.032 |
| Disease stage | |||
| II-III | Reference | ||
| IV | 0.31 | 0.06-1.66 | 0.173 |
| Distant metastasis-free survival | |||
| Treatment regimen status | |||
| CCRT | Reference | ||
| CTX/NTZ plus CCRT | 0.55 | 0.31-0.91 | 0.031 |
| Gender | |||
| Female | Reference | ||
| Male | 1.50 | 1.02-2.22 | 0.042 |
| Age | |||
| <45 | Reference | ||
| ≥45 | 1.08 | 0.79-1.46 | 0.639 |
| Karnofsky performance status score | |||
| 70-80 | Reference | ||
| 90-100 | 1.01 | 0.74-1.52 | 0.892 |
| Tumor stage | |||
| T1-T3 | Reference | ||
| T4 | 1.58 | 0.55-4.54 | 0.400 |
| Node stage | |||
| N0-N1 | Reference | ||
| N2 | 1.52 | 1.10-2.11 | 0.011 |
| N3 | 2.89 | 1.04-8.07 | 0.043 |
| Disease stage | |||
| II-III | Reference | ||
| IV | 1.31 | 0.43-3.93 | 0.635 |
| Overall survival | |||
| Treatment regimen status | |||
| CCRT | Reference | ||
| CTX/NTZ plus CCRT | 0.40 | 0.19-0.82 | 0.012 |
| Gender | |||
| Female | Reference | ||
| Male | 1.30 | 0.86-1.97 | 0.219 |
| Age | |||
| <45 | Reference | ||
| ≥45 | 1.44 | 1.02-2.02 | 0.039 |
| Karnofsky performance status score | |||
| 70-80 | Reference | ||
| 90-100 | 0.88 | 0.34-1.96 | 0.412 |
| Tumor stage | |||
| T1-T3 | Reference | ||
| T4 | 3.32 | 1.12-9.89 | 0.031 |
| Node stage | |||
| N0-N1 | Reference | ||
| N2 | 1.75 | 1.23-2.50 | 0.002 |
| N3 | 5.23 | 1.90-14.40 | 0.001 |
| Disease stage | |||
| II-III | Reference | ||
| IV | 0.64 | 0.20-2.02 | 0.443 |
The data originate from 1628 patients included in the study. HR, hazard ratio; 95% CI, 95% confidence interval.
Interaction between treatment regimen status and other significant prognostic factors and its effect on disease-free survival, distant metastasis-free survival, loco-regional relapse-free survival, and overall survival
| Disease-free survival | Loco-regional relapse-free survival | Distant metastasis-free survival | Overall survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted HRa (95% CI) | P value | Adjusted HRa (95% CI) | P value | Adjusted HRa (95% CI) | P value | Adjusted HRa (95% CI) | P value | ||||
| Treatment regimen status and Node Stage | |||||||||||
| Treatment regimen status | |||||||||||
| CCRT alone | Reference | Reference | Reference | Reference | |||||||
| CTX/NTZ plus CCRT | 0.43 (0.19-0.98) | 0.042 | 0.50 (0.16-1.61) | 0.247 | 0.40 (0.15-1.09) | 0.074 | 0.42 (0.13-1.32) | 0.137 | |||
| Node Stage | |||||||||||
| N0-N1 | Reference | Reference | Reference | Reference | |||||||
| N2 | 1.53 (1.12-2.10) | 0.008 | 1.36 (0.82-2.27) | 0.230 | 1.53 (1.06-2.20) | 0.023 | 1.87 (1.26-2.77) | 0.002 | |||
| N3 | 4.08 (1.71-9.72) | 0.002 | 4.84 (1.15-20.34) | 0.032 | 2.92 (1.05-8.14) | 0.041 | 5.46 (1.98-15.02) | 0.001 | |||
| Interaction Effect | |||||||||||
| CTX/NTZ plus CCRT * N2 | 1.66 (0.58-4.76) | 0.346 | 1.04 (0.20-5.48) | 0.967 | 1.69 (0.47-6.05) | 0.420 | 0.93 (0.20-4.37) | 0.929 | |||
| CTX/NTZ plus CCRT * N3 | 1.46 (0.27-7.90) | 0.662 | 2.30 (0.20-26.98) | 0.509 | 1.75 (0.29-10.42) | 0.540 | 1.05 (0.10-11.07) | 0.970 | |||
| Treatment regimen status and Tumor Stage | |||||||||||
| Treatment regimen status | |||||||||||
| CCRT alone | Reference | Reference | Reference | Reference | |||||||
| CTX/NTZ plus CCRT | 0.90 (0.19-4.34) | 0.893 | 0.30 (0.03-3.06) | 0.307 | 1.08 (0.16-7.12) | 0.936 | 0.46 (0.05-4.08) | 0.484 | |||
| Tumor Stage | |||||||||||
| T1-T3 | Reference | Reference | Reference | Reference | |||||||
| T4 | 2.79 (1.10-7.08) | 0.030 | 4.55 (0.92-22.48) | 0.063 | 1.58 (0.55-4.54) | 0.397 | 3.30 (1.11-9.84) | 0.032 | |||
| Interaction Effect | |||||||||||
| CTX/NTZ plus CCRT * T4 | 0.69 (0.19-2.47) | 0.563 | 1.65 (0.29-9.29) | 0.569 | 0.57 (0.12-2.70) | 0.480 | 0.90 (0.17-4.70) | 0.902 | |||
The data originate from all 1628 patients included in the study: HR, Hazard ratio; CI, confidence interval. a Multivariable cox regression model adjusted for age, gender, Karnofsky performance status score, tumor stage, node stage, disease stage
CTX in combination with standard cytotoxic therapies has shown consistent anticancer activity across a wide range of EGFR-expressing tumors, including NPC, squamous cell carcinoma of the head and neck (SCCHN), colorectal cancer, and non-small-cell lung cancer [12, 19-21]. Ma et al. conducted a phase II study of concurrent CTX-CDDP and IMRT in locoregionally advanced NPC and reported the 2-year progression-free survival rate of 86.5% with tolerable treatment-related toxicities. They also reported that concurrent administration of CTX, CDDP, and IMRT was a feasible strategy against locoregionally advanced NPC [13]. Baselga evaluated the efficacy and safety of CTX in combination with platinum-based chemotherapy in patients with platinum-refractory recurrent or metastatic SCCHN. They reported a disease control rate of 53% and the median time to progression and overall survival of 85 and 183 days, respectively, with well-tolerated treatment-related toxicities [22]. In addition, Anthony et al. published the results of a multicenter, phase II study in which they evaluated efficacy and toxicity of CTX plus carboplatin in recurrent or metastatic NPC resistant to platinum treatment. Overall response rate of 11.7%, the median time to progression and overall survival of 81 days and 233 days, respectively, were reported in this study [12].
It has been shown that CTX appears to overcome resistance to previously administered chemotherapy [20]. Also, CTX plus platinum-fluorouracil chemotherapy could further improve OS and DFS when given as first-line treatment in patients with recurrent or metastatic SCCHN compared with platinum-based chemotherapy plus fluorouracil alone [23]. Therefore, we postulated that the combination CTX and cisplatin-based chemoradiotherapy would kill tumor cells to a greater extent, especially the cisplatin-based chemotherapy resistant micro-metastases. This could partially explain the significant increase in DMFS of CTX/NTZ plus CCRT compared with CCRT alone in the current study. Our comparative analysis demonstrated that CTX/NTZ plus CCRT, as opposed to CCRT alone, was associated with a significantly better OS, DFS, DMFS, but not LRRFS. These data indicated that the increase in survival outcome for NPC patients treated with CTX/NTZ plus CCRT was mainly attributed to the significant increase in DMFS.
Although disease stage did not affect DFS, DMFS, and OS in the multivariate analysis, there were significant differences in the risks of disease progression, distant metastases, and death between stage II and stage IV in the univariate analysis. Due to the significant correlation between disease stage and T/N stage, the effect of disease stage on DFS, DMFS, and OS might be compromised by that of T/N stage in the multivariate analysis.
With the development of radiation techniques such as IMRT, patients can consistently receive a higher dose of radiation to the target tissue while sparing healthy organs at risk, thereby potentially enhancing the therapeutic efficacy. Previous studies have reported 90% control rates for nasopharyngeal carcinoma with the use of IMRT combined with systematic chemotherapy even in patients presenting with advanced loco-regional disease [4, 24]. Due to the advances in IMRT, there was no difference in the loco-regional relapse survival between CTX/NTZ plus CCRT and CCRT arms.
In the present study, the treatment outcomes in the chemoradiotherapy alone group were superior to those in similar treatment groups in previous trials using intensity-modulated radiotherapy [25, 26]. The reason for the better treatment outcome could be because more patients in this study had T1-T2/N0-N1M0 and stage II disease than stage IV disease. Importantly, multivariate analysis and interaction tests showed that the combination of CTX or NTZ with conventional CCRT was a significant protective factor for DMFS, DFS, and OS and was associated with a better survival outcome in patients in all stages II through IVb. This suggests that CTX/NTZ should be chosen together with CCRT in nasopharyngeal carcinoma patients regardless of their disease stage.
During concurrent chemoradiotherapy, 31.2% of patients in the CTX/NTZ plus CCRT arm and 30.8% of patients in the CCRT alone arm completed three cycles of concurrent cisplatin (P>0.05) indicating the addition of CTX/NTZ did not compromise the completion rate of concomitant CDDP. However, the completion rate of three cycles of concomitant CDDP in our study was lower than the results reported by Sun et al. [25] which may be due to the differences in the timing and treatment regimens between the two protocols. The proportion of patients receiving at least 200 mg/m2 of total administered concurrent cisplatin was high in both CTX/NTZ plus CCRT arm and CCRT arm (75.1% vs 72.7%, P>0.05). In terms of treatment-related toxicities, an increased rate of CTX related-skin reaction and mucositis was observed in the CTX plus CCRT arm. NTZ may, therefore, be an ideal alternative addition to the cisplatin based chemotherapy as it is not expected significantly increase treatment-related toxicities.
As for the limitations of this retrospective design, although we eliminated selection biases, such as gender, age, KPS, T and N stages, disease stage using propensity scores, it is unclear whether other confounding factors still exist. Also, this was a single-center, retrospective study originating in a high-prevalence area. In the future, a well-designed, multi-center, prospective, randomized study is needed to evaluate the efficacy and safety of concomitant CTX/NTZ and CCRT in NPC patients.
In conclusion, the addition of CTX/NTZ to chemoradiotherapy may be more effective for maximizing survival for patients with stage II-IVb NPC compared with chemoradiotherapy alone. However, more studies, especially prospective studies, are necessary to verify our findings.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.81572912), the New Century Excellent Talents in University (NCET-12-0562), Guangdong Public Welfare Research and Capacity Building Projects (2014B020212005), Guangdong Provincial Natural Science Foundation in China (S2013020012726), the Program of Sun Yat-Sen University for Clinical Research 5010 Program (No.201310 and No. 2015011), the Major Project of Sun Yat-Sen University for the New Cross Subject, and the Special Support Program for High-level Talents in Sun Yat-Sen University Cancer Center (to M.Y. Chen).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J cancer. 2011;30:114-9
2. National Comprehensive Cancer Network. NCCN Guidelines: Head and neck cancers; Version 2.2013. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp
3. Kam MK, Chau RM, Suen J, Choi PH, Teo PM. Intensity-modulated radiotherapy in nasopharyngeal carcinoma: dosimetric advantage over conventional plans and feasibility of dose escalation. Int J Radiat Oncol Biol Phys. 2003;56:145-57
4. Zhang MX, Li J, Shen GP, Zou X, Xu JJ, Jiang R. et al. Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: A 10-year experience with a large cohort and long follow-up. Eur J Cancer. 2015;51:2587-95
5. Lee AW, Sze WM, Au JS, Leung SF, Leung TW, Chua DT. et al. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2005;61:1107-16
6. Ma BB, Hui EP, Chan AT. Systemic approach to improving treatment outcome in nasopharyngeal carcinoma: current and future directions. Cancer Sci. 2008;99:1311-8
7. Teo PM, Kwan WH, Lee WY, Leung SF, Johnson PJ. Prognosticators determining survival subsequent to distant metastasis from nasopharyngeal carcinoma. Cancer. 1996;77:2423-31
8. Geara FB, Sanguineti G, Tucker SL, Garden AS, Ang KK, Morrison WH. et al. Carcinoma of the nasopharynx treated by radiotherapy alone: determinants of distant metastasis and survival. Radiother Oncol. 1997;43:53-61
9. Hui EP, Leung SF, Au JS, Zee B, Tung S, Chua D. et al. Lung metastasis alone in nasopharyngeal carcinoma: a relatively favorable prognostic group. A study by the Hong Kong Nasopharyngeal Carcinoma Study Group. Cancer. 2004;101:300-6
10. Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001;7:2958-70
11. Mendelsohn J. Targeting the epidermal growth factor receptor for cancer therapy. J Clin Oncol. 2002;20:1S-13S
12. Chan AT, Hsu MM, Goh BC, Hui EP, Liu TW, Millward MJ. et al. Multicenter, phase II study of cetuximab in combination with carboplatin in patients with recurrent or metastatic nasopharyngeal carcinoma. J Clin Oncol. 2005;23:3568-76
13. Ma BB, Kam MK, Leung SF, Hui EP, King AD, Chan SL. et al. A phase II study of concurrent cetuximab-cisplatin and intensity-modulated radiotherapy in locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. 2012;23:1287-92
14. Liu ZG, Zhao Y, Tang J, Zhou YJ, Yang WJ, Qiu YF. et al. Nimotuzumab combined with concurrent chemoradiotherapy in locally advanced nasopharyngeal carcinoma: a retrospective analysis. Oncotarget. 2016
15. Zhai RP, Ying HM, Kong FF, Du CR, Huang S, Zhou JJ. et al. Experience with combination of nimotuzumab and intensity-modulated radiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma. Onco Targets Ther. 2015;8:3383-90
16. Edge SB, Byrd DR. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-4
17. Tang LQ, Li CF, Li J, Chen WH, Chen QY, Yuan LX. et al. Establishment and Validation of Prognostic Nomograms for Endemic Nasopharyngeal Carcinoma. J Natl Cancer Inst. 2016:108
18. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-46
19. Pfister DG, Su YB, Kraus DH, Wolden SL, Lis E, Aliff TB. et al. Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: a pilot phase II study of a new combined-modality paradigm. J Clin Oncol. 2006;24:1072-8
20. Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A. et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-45
21. Yang ZY, Liu L, Mao C, Wu XY, Huang YF, Hu XF. et al. Chemotherapy with cetuximab versus chemotherapy alone for chemotherapy-naive advanced non-small cell lung cancer. Cochrane Database Syst Rev. 2014:CD009948
22. Baselga J, Trigo JM, Bourhis J, Tortochaux J, Cortes-Funes H, Hitt R. et al. Phase II multicenter study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2005;23:5568-77
23. Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S. et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116-27
24. Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P. et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol. 2009;27:3684-90
25. Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY. et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17:1509-20
26. Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y. et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2012;13:163-71
Author contact
![]() Corresponding author: Prof. Ming-Yuan Chen, Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, 651 Dongfeng East Road, Guangzhou, Guangdong 510060, P. R. China E-mail: chmingysysu.edu.cn; Phone: 86-20-8734-3361; Fax: 86-20-87343624
Corresponding author: Prof. Ming-Yuan Chen, Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, 651 Dongfeng East Road, Guangzhou, Guangdong 510060, P. R. China E-mail: chmingysysu.edu.cn; Phone: 86-20-8734-3361; Fax: 86-20-87343624
 Global reach, higher impact
Global reach, higher impact