13.3
Impact Factor
Theranostics 2017; 7(8):2325-2338. doi:10.7150/thno.18225 This issue Cite
Research Paper
Atg9b Deficiency Suppresses Autophagy and Potentiates Endoplasmic Reticulum Stress-Associated Hepatocyte Apoptosis in Hepatocarcinogenesis
School of Chinese Medicine, The University of Hong Kong, Hong Kong S.A.R, PR of China
Abstract
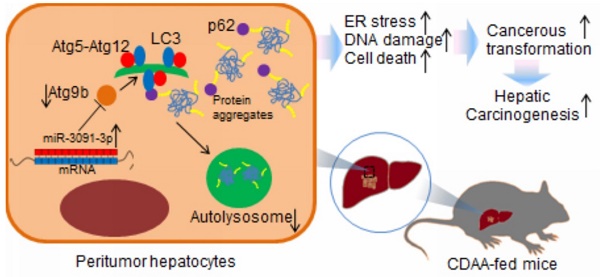
The aim of this study was to investigate the mechanism underlying autophagy deficiency during hepatic carcinogenesis. For this purpose, we used choline-deficient, amino acid-defined (CDAA) hepatocarcinogenesis model in mice. miRNA microarrays combined with computational target predictions and GO analysis were used to identify molecular processes involved in carcinogenesis. PCR profiler array was employed to detect the dysregulated autophagy-related genes during carcinogenesis. We observed induction of hepatic tumours with increased inflammation, DNA damage, and cell death. These cellular processes were particularly detected upon oncogenic transformation of hepatocytes in which ER stress was excessively induced. Microarray combined with GO analysis showed that transformation of hepatocytes resulted in dysregulated events associated with cytoplasmic vesicle formation, which, in turn, was related to ER stress-induced autophagy. Defects of autophagy were observed in livers harbouring tumours and suffered a loss of expression of autophagy-related protein 9b (Atg9b). Hepatocytes lacking Atg9b were vulnerable to cell death induced by ER stress stimulus mainly caused by accumulation of ubiquitinated proteins. Loss of Atg9b also blocked recruitment of p62-associated ubiquitinated protein for autophagosome-lysosome degradation as Atg9b-driven phagophores may facilitate docking of both LC3 and p62 to initiate autophagy-associated degradation. miR-3091-3p from tumour-derived exosomes, which were internalised by hepatocytes, could suppress Atg9b expression. Observations from this study advance our knowledge about the regulation of autophagy during hepatocarcinogenesis.
Keywords: Hepatic carcinogenesis, Autophagy, Atg9b, ER stress, Tumour-derived exosome, microRNA.
 Global reach, higher impact
Global reach, higher impact