13.3
Impact Factor
Theranostics 2017; 7(9):2402-2416. doi:10.7150/thno.17994 This issue Cite
Research Paper
In-depth Characterization of a TCR-specific Tracer for Sensitive Detection of Tumor-directed Transgenic T Cells by Immuno-PET
1. Department of Nuclear Medicine, Klinikum rechts der Isar, Technische Universität München, Germany;
2. III. Medical Department, Klinikum rechts der Isar, Technische Universität München, Germany;
3. German Cancer Consortium (DKTK), partner site Munich and German Cancer Research Center (DKFZ), Heidelberg, Germany;
4. Institute of Pathology Technische Universität München, Germany;
5. Comparative Experimental Pathology, Technische Universität München, Germany.
* Joint senior authorship
Abstract
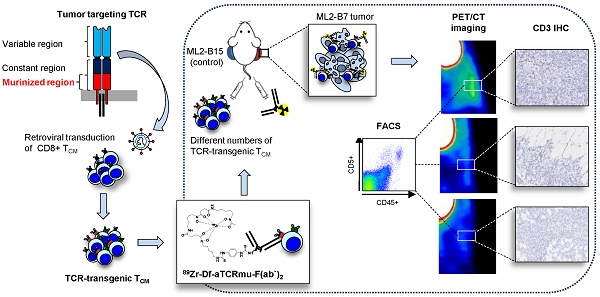
A number of different technologies have been developed to monitor in vivo the distribution of gene-modified T cells used in immunotherapy. Nevertheless, in-depth characterization of novel approaches with respect to sensitivity and clinical applicability are so far missing. We have previously described a novel method to track engineered human T cells in tumors using 89Zr-Df-aTCRmu-F(ab')2 targeting the murinized part of the TCR beta domain (TCRmu) of a transgenic TCR. Here, we performed an in-depth in vitro characterization of the tracer in terms of antigen affinity, immunoreactivity, influence on T-cell functionality and stability in vitro and in vivo. Of particular interest, we have developed diverse experimental settings to quantify TCR-transgenic T cells in vivo. Local application of 89Zr-Df-aTCRmu-F(ab')2-labeled T cells in a spot-assay revealed signal detection down to approximately 1.8x104 cells. In a more clinically relevant model, NSG mice were intravenously injected with different numbers of transgenic T cells, followed by injection of the 89Zr-Df-aTCRmu-F(ab')2 tracer, PET/CT imaging and subsequent ex vivo T-cell quantification in the tumor. Using this setting, we defined a comparable detection limit of 1.0x104 T cells. PET signals correlated well to total numbers of transgenic T cells detected ex vivo independently of the engraftment rates observed in different individual experiments. Thus, these findings confirm the high sensitivity of our novel PET/CT T-cell tracking method and provide critical information about the quantity of transgenic T cells in the tumor environment suggesting our technology being highly suitable for further clinical translation.
Keywords: In vivo T-cell imaging, immuno-PET, T-cell quantification, cancer immunotherapy, T-cell receptor (TCR)-transgenic T cells.
 Global reach, higher impact
Global reach, higher impact