13.3
Impact Factor
Theranostics 2017; 7(13):3260-3275. doi:10.7150/thno.19979 This issue Cite
Research Paper
Direct Macromolecular Drug Delivery to Cerebral Ischemia Area using Neutrophil-Mediated Nanoparticles
1. Department of Pharmaceutics, School of Pharmacy, Fudan University & Key Laboratory of Smart Drug Delivery, Ministry of Education, Shanghai 201203, PR China;
2. Institute of Clinical Pharmacology, Guangzhou University of Traditional Chinese Medicine, Guangzhou 510006, PR China;
3. School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu 610072, PR China;
4. Key Laboratory of Drug Targeting and Drug Delivery System, Ministry of Education (Sichuan University).
Abstract
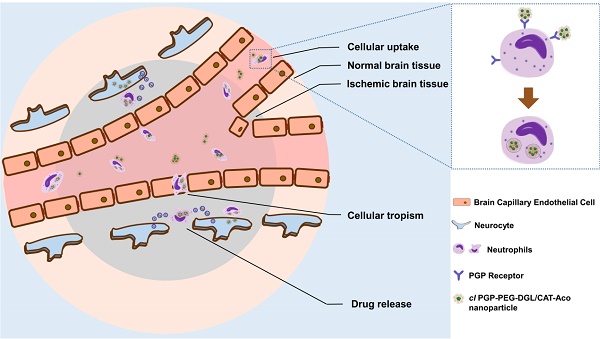
Delivery of macromolecular drugs to the brain is impeded by the blood brain barrier. The recruitment of leukocytes to lesions in the brain, a typical feature of neuroinflammation response which occurs in cerebral ischemia, offers a unique opportunity to deliver drugs to inflammation sites in the brain. In the present study, cross-linked dendrigraft poly-L-lysine (DGL) nanoparticles containing cis-aconitic anhydride-modified catalase and modified with PGP, an endogenous tripeptide that acts as a ligand with high affinity to neutrophils, were developed to form the cl PGP-PEG-DGL/CAT-Aco system. Significant binding efficiency to neutrophils, efficient protection of catalase enzymatic activity from degradation and effective transport to receiver cells were revealed in the delivery system. Delivery of catalase to ischemic subregions and cerebral neurocytes in MCAO mice was significantly enhanced, which obviously reducing infarct volume in MCAO mice. Thus, the therapeutic outcome of cerebral ischemia was greatly improved. The underlying mechanism was found to be related to the inhibition of ROS-mediated apoptosis. Considering that neuroinflammation occurs in many neurological disorders, the strategy developed here is not only promising for treatment of cerebral ischemia but also an effective approach for various CNS diseases related to inflammation.
Keywords: brain targeting, catalase, neutrophils, PGP, ischemic stroke.
 Global reach, higher impact
Global reach, higher impact