13.3
Impact Factor
Theranostics 2018; 8(2):505-517. doi:10.7150/thno.21967 This issue Cite
Research Paper
Magnetic Nanowire Networks for Dual-Isolation and Detection of Tumor-Associated Circulating Biomarkers
1. Biomarker Branch, National Cancer Center, 323 Ilsan-ro, Ilsan-dong-gu, Goyang, Gyeonggi 10408, Korea;
2. Center for Lung Cancer, National Cancer Center, 323 Ilsan-ro, Ilsan-dong-gu, Goyang, Gyeonggi 10408, Korea;
3. Department of Cancer Biomedical Science, Graduate School of Cancer Science and Policy, 323 Ilsan-ro, Ilsan-dong-gu, Goyang, Gyeonggi 10408, Korea;
4. Department of Medical Science, Yonsei University College of Medicine, 50 Yonsei-Ro, Seodaemun-Gu, Seoul 03722, South Korea;
5. Department of Internal Medicine, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul 03080, Korea;
6. Genopsy Inc., HongWoo BD 103-B, 373, Kangnamdaero, Seocho-Gu, Seoul 06621, Korea.
* These authors contributed equally to this work.
Abstract
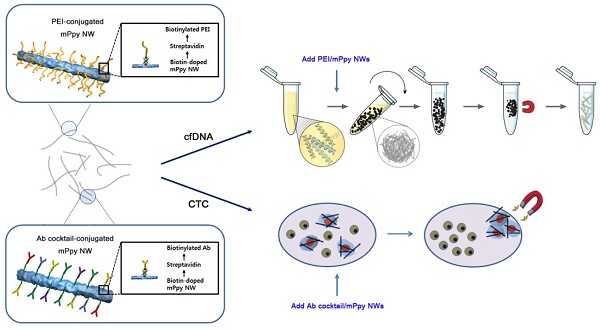
Purpose: Recent developments in genomic and molecular methods have revolutionized the range of utilities of tumor-associated circulating biomarkers in both basic and clinical research. Herein, we present a novel approach for ultrasensitive extraction of cfDNA and CTCs, at high yield and purity, via the formation of magnetic nanowire networks.
Materials and Methods: We fabricated and characterized biotinylated cationic polyethylenimine and biotinylated antibody cocktail-conjugated magnetic polypyrrole NWs (PEI/mPpy NW and Ab cocktail/mPpy NW, respectively). We applied these NWs to the extraction of cfDNA and CTC from the blood of 14 patients with lung cancer. We demonstrated reliable detection of EGFR mutations based on digital droplet PCR analysis of cfDNA and CTC DNA from patients with lung cancer.
Results: The NW networks confined with a high density of magnetic nanoparticles exhibited superior saturation magnetization, which enabled rapid and high-yield capture whilst avoiding or minimizing damage and loss. The NW networks enabled the co-isolation of CTCs and cfDNA of high quality and sufficient quantities, thus allowing the amplification of rare and low-prevalence cancer-related mutations.
Conclusion: The simple, versatile, and highly efficient nanowire network tool allows sensitive and robust assessment of clinical samples.
Keywords: Circulating cell-free DNA, circulating tumor cells, lung cancer, blood, plasma, conducting polymer, nanowire.
 Global reach, higher impact
Global reach, higher impact