13.3
Impact Factor
Theranostics 2018; 8(9):2459-2476. doi:10.7150/thno.23880 This issue Cite
Research Paper
Endogenous IgG-based affinity-controlled release of TRAIL exerts superior antitumor effects
1. Key Lab of Transplant Engineering and Immunology, MOH;
2. Department of Nuclear Medicine;
3. Proteomics and Metabolomics Laboratory, West China-Washington Mitochondria and Metabolism Research Center; West China Hospital, Sichuan University, Chengdu, 610041, China
4. Present address: College of Pharmacy, Liaocheng University, Liaocheng, Shandong, 252000, China
*These authors contribute equally to this work.
Abstract
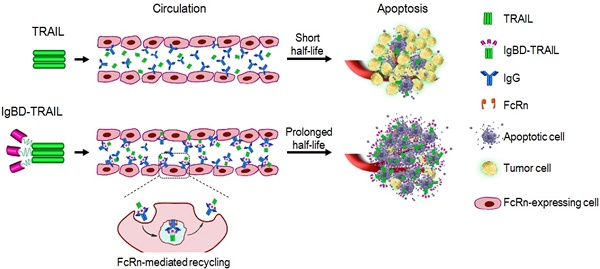
The inefficiency of recombinant tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-based clinical regimens has been dominantly attributed to the short half-life of TRAIL. Affinity-controlled release using endogenous long-acting proteins, such as IgG and albumin, as carriers is extremely attractive for improving the pharmacokinetics of TRAIL. Up to now, it is unclear whether IgG-binding is efficient for affinity-controlled release of TRAIL.
Methods: An IgG-binding affibody, IgBD, was genetically fused to the N-terminus of TRAIL to produce IgBD-TRAIL.The IgG-binding ability, cytotoxicity, serum half-life, and in vivo antitumor effect of IgBD-TRAIL were compared with that of TRAIL. In addition, an albumin-binding affibody, ABD, was fused to TRAIL to produce ABD-TRAIL. The cytototoxicity, serum half-life, and antitumor effect of IgBD-TRAIL and ABD-TRAIL were compared.
Results: IgBD fusion endowed TRAIL with high affinity (nM) for IgG without interference with its cytotoxicity. The serum half-life of IgBD-TRAIL is 50-60 times longer than that of TRAIL and the tumor uptake of IgBD-TRAIL at 8-24 h post-injection was 4-7-fold that of TRAIL. In vivo antitumor effect of IgBD-TRAIL was at least 10 times greater than that of TRAIL. Owing to the high affinity (nM) for albumin, the serum half-life of ABD-TRAIL was 80-90 times greater than that of TRAIL. However, after binding to albumin, the cytotoxicity of ABD-TRAIL was reduced more than 10 times. In contrast, binding to IgG had little impact on the cytotoxicity of IgBD-TRAIL. Consequently, intravenously injected IgBD-TRAIL showed antitumor effects superior to those of ABD-TRAIL.
Conclusions: Endogenous long-acting proteins, particularly IgG-based affinity-controlled release, prolonged the serum half-life as well as significantly enhanced the antitumor effect of TRAIL. IgBD-mediated endogenous IgG binding might be a novel approach for the affinity-controlled release of other protein drugs.
Keywords: drug delivery, affinity-controlled release, biopharmaceuticals, immunoglobulin, albumin
 Global reach, higher impact
Global reach, higher impact