13.3
Impact Factor
Theranostics 2018; 8(9):2508-2520. doi:10.7150/thno.25599 This issue Cite
Research Paper
First-in-human study of PET and optical dual-modality image-guided surgery in glioblastoma using 68Ga-IRDye800CW-BBN
1. Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
2. Department of Nuclear Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
3. Key Laboratory of Molecular Imaging, Chinese Academy of Science, Beijing, China
4. China National Clinical Research Center for Neurological Diseases (NCRC-ND), Beijing, China
5. Department of Neuropathology, Beijing Neurosurgical Institute, Capital Medical University, Beijing, China
6. Laboratory of Molecular Imaging and Nanomedicine (LOMIN), National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (NIH), Bethesda, Maryland, United States
*These authors contributed equally to this work.
Abstract
Purpose: Despite the use of fluorescence-guided surgery (FGS), maximum safe resection of glioblastoma multiforme (GBM) remains a major challenge. It has restricted surgeons between preoperative diagnosis and intraoperative treatment. Currently, an integrated approach combining preoperative assessment with intraoperative guidance would be a significant step in this direction.
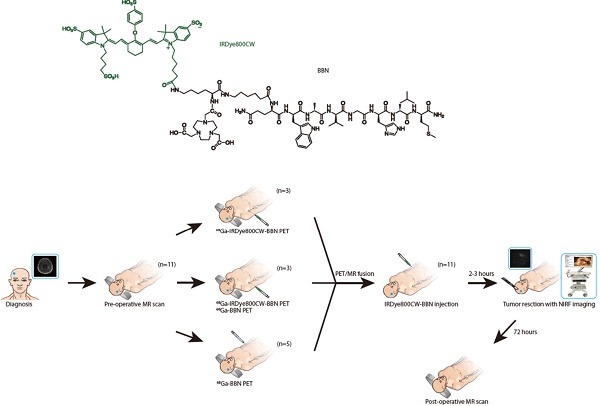
Experimental design: We developed a novel 68Ga-IRDye800CW-BBN PET/near-infrared fluorescence (NIRF) dual-modality imaging probe targeting gastrin-releasing peptide receptor (GRPR) in GBM. The preclinical in vivo tumor imaging and FGS were first evaluated using an orthotopic U87MG glioma xenograft model. Subsequently, the first-in-human prospective cohort study (NCT 02910804) of GBM patients were conducted with preoperative PET assessment and intraoperative FGS.
Results: The orthotopic tumors in mice could be precisely resected using the near-infrared intraoperative system. Translational cohort research in 14 GBM patients demonstrated an excellent correlation between preoperative positive PET uptake and intraoperative NIRF signal. The tumor fluorescence signals were significantly higher than those from adjacent brain tissue in vivo and ex vivo (p < 0.0001). Compared with pathology, the sensitivity and specificity of fluorescence using 42 loci of fluorescence-guided sampling were 93.9% (95% CI 79.8%-99.3%) and 100% (95% CI 66.4%-100%), respectively. The tracer was safe and the extent of resection was satisfactory without newly developed neurologic deficits. Progression-free survival (PFS) at 6 months was 80% and two newly diagnosed patients achieved long PFS.
Conclusions: This initial study has demonstrated that the novel dual-modality imaging technique is feasible for integrated pre- and intraoperative targeted imaging via the same molecular receptor and improved intraoperative GBM visualization and maximum safe resection.
Keywords: glioblastoma, dual-modality imaging, positron emission tomography (PET), near-infrared fluorescence, intraoperative imaging, gastrin-releasing peptide receptor
 Global reach, higher impact
Global reach, higher impact