13.3
Impact Factor
Theranostics 2018; 8(14):3902-3917. doi:10.7150/thno.24444 This issue Cite
Research Paper
Profiling the circulating miRnome reveals a temporal regulation of the bone injury response
1. i3S - Instituto de Investigação e Inovação em Saúde, INEB - Instituto de Engenharia Biomédica, Universidade do Porto, Porto, Portugal.
2. ICBAS - Instituto de Ciências Biomédicas Abel Salazar, Universidade do Porto, Porto, Portugal.
3. Department of Experimental Therapeutics, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
4. FEUP - Faculdade de Engenharia da Universidade do Porto, Porto, Portugal.
5. FMUP - Faculdade de Medicina da Universidade do Porto, Departamento de Cirurgia, Serviço de Ortopedia, Porto, Portugal.
6. Center for RNA Interference and Non-Coding RNAs, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Abstract
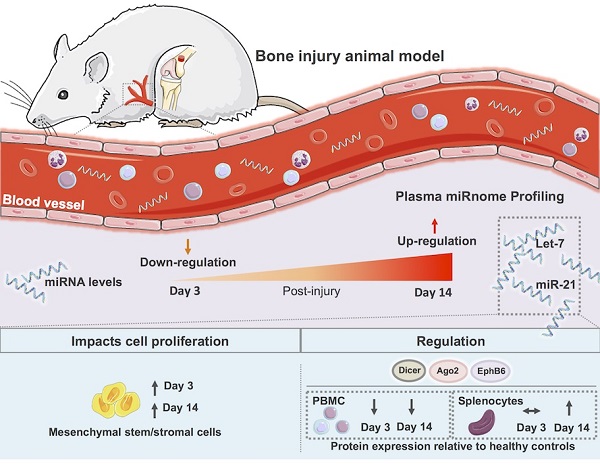
Bone injury healing is an orchestrated process that starts with an inflammatory phase followed by repair and remodelling of the bone defect. The initial inflammation is characterized by local changes in immune cell populations and molecular mediators, including microRNAs (miRNAs). However, the systemic response to bone injury remains largely uncharacterized. Thus, this study aimed to profile the changes in the plasma miRnome after bone injury and determine its biological implications.
Methods: A rat model of femoral bone defect was used, and animals were evaluated at days 3 and 14 after injury. Non-operated (NO) and sham operated animals were used as controls. Blood and spleen were collected and peripheral blood mononuclear cells (PBMC) and plasma were separated. Plasma miRnome was determined by RT-qPCR array and bioinformatics Ingenuity pathway analysis (IPA) was performed. Proliferation of bone marrow mesenchymal stem/stromal cells (MSC) was evaluated by Ki67 staining and high-throughput cell imaging. Candidate miRNAs were evaluated in splenocytes by RT-qPCR, and proteins found in the IPA analysis were analysed in splenocytes and PBMC by Western blot.
Results: Bone injury resulted in timely controlled changes to the miRNA expression profile in plasma. At day 3 there was a major down-regulation of miRNA levels, which was partially recovered by day 14 post-injury. Interestingly, bone injury led to a significant up-regulation of let-7a, let-7d and miR-21 in plasma and splenocytes at day 14 relative to day 3 after bone injury, but not in sham operated animals. IPA predicted that most miRNAs temporally affected were involved in cellular development, proliferation and movement. MSC proliferation was analysed and found significantly increased in response to plasma of animals days 3 and 14 post-injury, but not from NO animals. Moreover, IPA predicted that miRNA processing proteins Ago2 and Dicer were specifically inhibited at day 3 post-injury, with Ago2 becoming activated at day 14. Protein levels of Ago2 and Dicer in splenocytes were increased at day 14 relative to day 3 post-bone injury and NO animals, while in PBMC, levels were reduced at day 3 (albeit Dicer was not significant) and remained low at day 14. Ephrin receptor B6 followed the same tendency as Ago2 and Dicer, while Smad2/3 was significantly decreased in splenocytes from day 14 relative to NO and day 3 post-bone injury animals.
Conclusion: Results show a systemic miRNA response to bone injury that is regulated in time and is related to inflammation resolution and the start of bone repair/regeneration, unravelling candidate miRNAs to be used as biomarkers in the monitoring of healthy bone healing and as therapeutic targets for the development of improved bone regeneration therapies.
Keywords: inflammation, bone tissue repair, plasma, microRNA, biomarker
 Global reach, higher impact
Global reach, higher impact