13.3
Impact Factor
Theranostics 2018; 8(15):4016-4032. doi:10.7150/thno.25958 This issue Cite
Review
Aptamers in the Therapeutics and Diagnostics Pipelines
1. AstraZeneca USA.
2. Operational Technologies Corporation, 4100 NW Loop 410, Suite 100, San Antonio, Texas 78229, USA.
3. Discipline of Biosciences and Biomedical Engineering, Indian Institute of Technology Indore, Simrol, Indore, 453552, India.
4. Center for Biodesign and Diagnostics, Translational Health Science and Technology Institute (THSTI), Faridabad-121001, Haryana, India.
Abstract
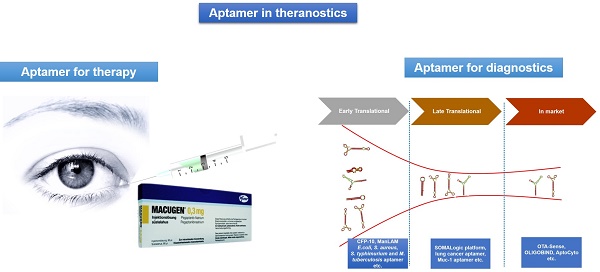
Aptamers are short single-stranded DNA or RNA oligonucleotides that can selectively bind to small molecular ligands or protein targets with high affinity and specificity, by acquiring unique three-dimensional structures. Aptamers have the advantage of being highly specific, relatively small in size, non-immunogenic and can be easily stabilized by chemical modifications, thus allowing expansion of their diagnostic and therapeutic potential. Since the invention of aptamers in the early 1990s, great efforts have been made to make them clinically relevant for diseases like macular degeneration, cancer, thrombosis and inflammatory diseases. Furthermore, owing to the aforementioned advantages and unique adaptability of aptamers to point-of-care platforms, aptamer technology has created a stable niche in the field of in vitro diagnostics by enhancing the speed and accuracy of diagnoses. The aim of this review is to give an overview on aptamers, highlight the inherent therapeutic and diagnostic opportunities and challenges associated with them and present various aptamers that have reached therapeutic clinical trials, diagnostic markets or that have immediate translational potential for therapeutics and diagnostics applications.
Keywords: aptamers, therapeutics, diagnostics, clinical trials, clinical diagnostics
 Global reach, higher impact
Global reach, higher impact