13.3
Impact Factor
Theranostics 2018; 8(15):4062-4071. doi:10.7150/thno.24683 This issue Cite
Research Paper
DNA nanotriangle-scaffolded activatable aptamer probe with ultralow background and robust stability for cancer theranostics
State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Biology, College of Chemistry and Chemical Engineering, Hunan University, Key Laboratory for Bio-Nanotechnology and Molecular Engineering of Hunan Province, Changsha 410082, China.
Abstract
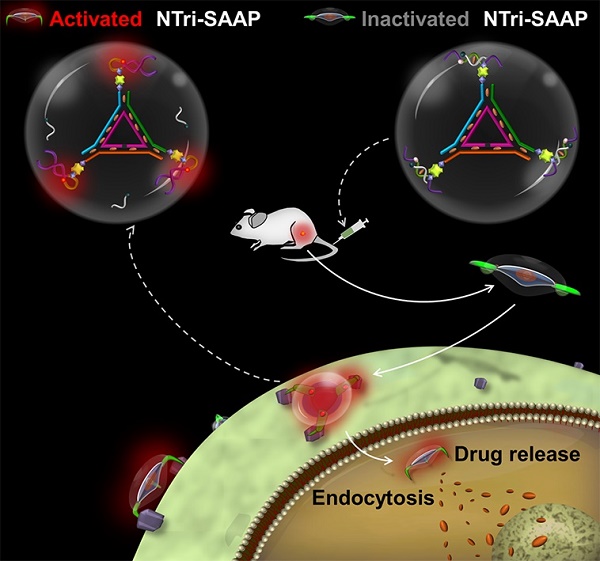
Activatable aptamers have emerged as promising molecular tools for cancer theranostics, but reported monovalent activatable aptamer probes remain problematic due to their unsatisfactory affinity and poor stability. To address this problem, we designed a novel theranostic strategy of DNA nanotriangle-scaffolded multivalent split activatable aptamer probe (NTri-SAAP), which combines advantages of programmable self-assembly, multivalent effect and target-activatable architecture.
Methods: NTri-SAAP was assembled by conjugating multiple split activatable aptamer probes (SAAPs) on a planar DNA nanotriangle scaffold (NTri). Leukemia CCRF-CEM cell line was used as the model to investigate its detection, imaging and therapeutic effect both in vitro and in vivo. Binding affinity and stability were evaluated using flow cytometry and nuclease resistance assays.
Results: In the free state, NTri-SAAP was stable with quenched signals and loaded doxorubicin, while upon binding to target cells, it underwent a conformation change with fluorescence activation and drug release after internalization. Compared to monovalent SAAP, NTri-SAAP displayed greatly-improved target binding affinity, ultralow nonspecific background and robust stability in harsh conditions, thus affording contrast-enhanced tumor imaging within an extended time window of 8 h. Additionally, NTri-SAAP increased doxorubicin loading capacity by ~5 times, which further realized a high anti-tumor efficacy in vivo with 81.95% inhibition but no obvious body weight loss.
Conclusion: These results strongly suggest that the biocompatible NTri-SAAP strategy would provide a promising platform for precise and high-quality theranostics.
Keywords: DNA self-assembly, multivalent effect, activatable imaging, split aptamer, cancer theranostics
 Global reach, higher impact
Global reach, higher impact