13.3
Impact Factor
Theranostics 2018; 8(17):4795-4804. doi:10.7150/thno.26093 This issue Cite
Research Paper
Inhibition of Notch1 induces population and suppressive activity of regulatory T cell in inflammatory arthritis
1. School of Pharmacy, Sungkyunkwan University, Suwon, Korea
2. Department of Pharmacology, Ajou University School of Medicine, Suwon, Korea
3. Department of Health Science and Technology, Samsung Advanced Institute for Health Science and Technology, Sungkyunkwan University, Seoul, Korea
4. Department of Physiology, Yong Loo Lin School Medicine, National University of Singapore, Singapore
5. Department of Life Science, Ewha Womans University, Seoul, Korea.
6. Department of Internal Medicine (Rheumatology), Kyungpook National University School of Medicine, Daegu, Korea
7. Department of Chemical Engineering, Hanyang University, Ansan, Korea.
8. College of Engineering, Sungkyunkwan University, Suwon, Korea
9. Biomedical Institute for Convergence, Sungkyunkwan University, Suwon, Korea
*Equal contribution.n
Abstract
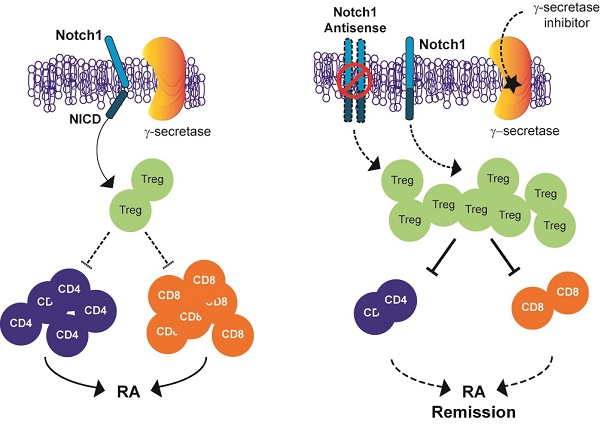
Inhibition of Notch signalling has shown anti-inflammatory properties in vivo and in vitro models of rheumatoid arthritis (RA). The objective of this study was to determine whether Notch1 might play a role in regulating T-regulatory cells (Tregs) in animal models of RA.
Methods: Collagen-induced arthritis (CIA) and collagen antibody-induced arthritis (CAIA) were induced in C57BL/6, Notch1 antisense transgenic (NAS) or DBA1/J mice. We examined whether pharmacological inhibitors of γ-secretase (an enzyme required for Notch1 activation) and antisense-mediated knockdown of Notch1 could attenuate the severity of inflammatory arthritis in CIA and CAIA mice. Proportions of CD4+CD25+Foxp3+ Treg cells were measured by flow cytometry. To assess the suppressive capacity of Treg toward responder cells, CFSE-based suppression assay of Treg was performed.
Results: γ-secretase inhibitors and antisense-mediated knockdown of Notch1 reduced the severity of inflammatory arthritis in both CIA and CAIA mice. Pharmacological and genetic inhibition of Notch1 signalling induced significant elevation of Treg cell population in CIA and CAIA mice. We also demonstrated that inhibition of Notch signalling suppressed the progression of inflammatory arthritis through modulating the expansion and suppressive function of regulatory T (Treg) cells.
Conclusion: Pharmacological and genetic inhibition of Notch1 signalling suppresses the progression of inflammatory arthritis through modulating the population and suppressive function of Treg cells in animal models of RA.
Keywords: rheumatoid arthritis, Notch1, Treg, CIA, CAIA, γ-secretase
 Global reach, higher impact
Global reach, higher impact