13.3
Impact Factor
Theranostics 2018; 8(19):5231-5245. doi:10.7150/thno.25286 This issue Cite
Research Paper
Photodynamic therapy at ultra-low NIR laser power and X-Ray imaging using Cu3BiS3 nanocrystals
1. Bio-Nano Electronics Research Centre, Toyo University, Kawagoe, 350-8585, Japan
2. Graduate School of Interdisciplinary New Science, Toyo University, Kawagoe, 350-8585, Japan
3. JEOL Ltd. Otemachi Nomura Bldg.13F, 2-1-1, Otemachi, Chiyoda, Tokyo, 100-0004, Japan
4. Biomedical Research Centre, Division of Analytical Science, Saitama Medical University, Saitama 350-0495, Japan
* Equal contributing Authors
Abstract
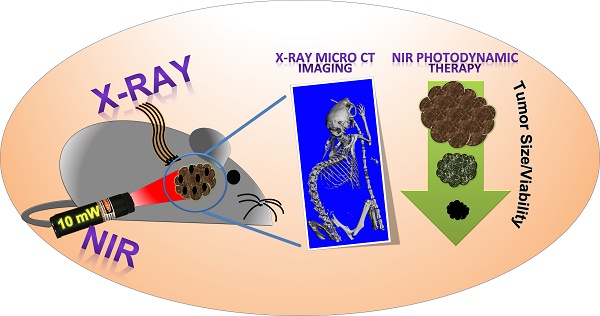
Materials with efficient potential in imaging as well as therapy are gaining particular attention in current medical research. Photodynamic therapy (PDT) has been recently recognized as a promising treatment option for solid tumors. Still, most of the nanomaterial-based PDT modules either employ an additional photosensitizer or require high power laser sources. Also, they suffer from a lack of responsiveness in the near-infrared (NIR) region. Nanomaterials that could realize PDT independently (without any photosensitizer), at safe laser dose and in the deep tissue penetrative NIR region would definitely be better solid tumor treatment options.
Methods: Herein, Cu- and Bi-based bimetal chalcogenide (Cu3BiS3), with absorption in the NIR region was developed. High-performance PDT of cancer and high-contrast x-ray imaging of tumor were performed in vivo. Biocompatibility of the NCs was also assessed in vivo.
Results: The highlight of the results was the realization of ultra-low dose NIR laser-mediated PDT, which has not been achieved before, leading to complete tumor regression. This could be a breakthrough in providing a pain- and scar-less treatment option, especially for solid tumors and malignant/benign subcutaneous masses. Though the NCs are active in the photo-thermal therapy (PTT) regime as well, focus is given to the exciting aspect of extremely low power-induced PDT observed here.
Conclusion: Their extended in vivo biodistribution with commendable hemo- and histo-compatibilities, along with imaging and multi-therapeutic capabilities, project these Cu3BiS3 NCs as promising, prospective theranostic candidates.
Keywords: photodynamic therapy, x-ray contrast imaging, reactive oxygen species, near-infrared laser, anticancer therapy
 Global reach, higher impact
Global reach, higher impact