13.3
Impact Factor
Theranostics 2018; 8(19):5362-5378. doi:10.7150/thno.27631 This issue Cite
Research Paper
A Biomimetic Gold Nanocages-Based Nanoplatform for Efficient Tumor Ablation and Reduced Inflammation
1. National Engineering Research Center for Nanomedicine, Hubei Key Laboratory of Bioinorganic Chemistry and Materia Medica, College of Life Science and Technology, Huazhong University of Science and Technology, Wuhan 430074, China
2. Britton Chance Center for Biomedical Photonics, Wuhan National Laboratory for Optoelectronics & Moe Key Laboratory of Biomedical Photonics of Ministry of Education, Department of Biomedical Engineering, Huazhong University of Science and Technology, Wuhan 430074, China
Abstract
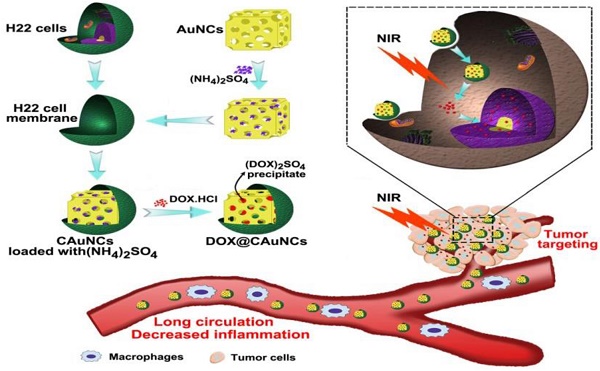
Gold nanocages (AuNCs), with high photothermal conversion efficiency and unique hollow interiors, have become a promising nanoplatform for synergistic phototheraml therapy (PTT)-chemotherapy. However, the insufficient tumor targeting, in vivo premature drug leakage and low drug loading efficiency responsible for the spatial-temporal un-synchronization of PTT-chemotherapy, as well as inflammatory response might compromise the anticancer treatment of AuNCs-based drug delivery systems.
Methods: Cancer cell membrane (CCM)-coated AuNCs were developed to load anticancer drug doxorubicin (DOX@CAuNCs) by transmembrane ammonium sulfate gradient method. In vitro and in vivo analysis, including characterization, macrophage phagocytosis and tumor targeting capacity, near-infrared (NIR) laser-induced drug release, antitumor efficacy and inflammation response were systematically performed.
Results: DOX@CAuNCs showed a high DOX loading capacity and on-demand NIR laser-triggered DOX release compared with CAuNCs passively loading DOX by electrostatic adsorption, a commonly used method to load drug to AuNCs. Meanwhile, in view of the properties of CCM coated on AuNCs, DOX@CAuNCs exhibited decreased macrophage phagocytosis, prolonged blood circulation and enhanced internalization by cancer cells, generating preferable tumor targeting ability. With these integrated advantages, DOX@CAuNCs demonstrated highly efficient and precise spatial-temporal synchronization of PTT-chemotherapy, achieving complete tumor ablation with no obvious side effects. Besides, coating with CCM significantly alleviated AuNCs-induced inflammatory response.
Conclusion: This biomimetic AuNCs-based platform might be a prospective drug delivery system for precision PTT and chemotherapy, acquiring desired cancer treatment efficacy and low inflammatory response.
Keywords: Gold nanocages, cancer cell membrane, high drug loading efficiency, spatial-temporal synchronization of photothermal-chemotherapy, inflammation, cancer therapy
 Global reach, higher impact
Global reach, higher impact