13.3
Impact Factor
Theranostics 2018; 8(19):5419-5433. doi:10.7150/thno.27876 This issue Cite
Research Paper
Exosomal microRNA-21 derived from bronchial epithelial cells is involved in aberrant epithelium-fibroblast cross-talk in COPD induced by cigarette smoking
1. Institute of Toxicology, School of Public Health, Nanjing Medical University, Nanjing 211166, Jiangsu, People's Republic of China.
2. The Key Laboratory of Modern Toxicology, Ministry of Education, School of Public Health, Nanjing Medical University, Nanjing 211166, Jiangsu, People's Republic of China.
3. Jiangsu Provincial Center for Disease Control and Prevention, Nanjing 210009, Jiangsu, People's Republic of China.
4. Liyang Center for Disease Control and Prevention, Liyang 213300, Jiangsu, People's Republic of China.
5. Faculty of Public Health, School of Medicine, Shanghai Jiaotong University, Shanghai 200025, People's Republic of China.
* Theses authors contributed equally to this work.
Abstract
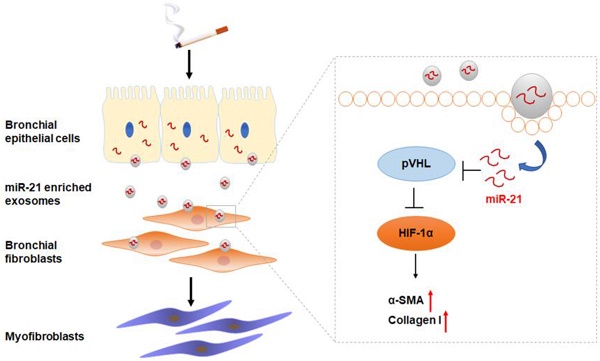
Rationale: Aberrant bronchial epithelium-fibroblast communication is essential for the airway remodeling that contributes to chronic obstructive pulmonary disease (COPD). Exosomes have emerged as novel mediators of intercellular communication, but their role in cigarette smoke (CS)-induced COPD is unknown. Here, we investigated the role of exosomal miR-21 in the dysfunctional epithelium-fibroblast cross-talk caused by CS.
Methods: Normal or CS extract (CSE)-treated human bronchial epithelial (HBE) cells were co-cultured with bronchial fibroblasts (MRC-5 cells). Exosomes were obtained from culture media or serum by use of commercial kits. The size distribution and concentration of exosomes were analyzed by nanoparticle tracking analysis using a ZetaView particle tracker from ParticleMetrix. Inhibition of miR-21 levels by tail vein injection of antagomir-21 into mice exposed to CS was used to demonstrate the role of miR-21 in airway remodeling leading to COPD in animals.
Results: For MRC-5 cells, co-culture with CSE-treated HBE cells or with exosomes derived from CSE-treated HBE cells resulted in the myofibroblast differentiation phenotype. Exosomal miR-21 was responsible for myofibroblast differentiation through hypoxia-inducible factor 1α (HIF-1α) signaling by targeting the von Hippel-Lindau protein (pVHL); HIF-1α transcriptionally regulated the α-SMA gene. For mice, downregulation of miR-21 prevented CS-induced airway remodeling. The levels of exosomal miR-21 were high in sera of smokers and COPD patients and inversely correlated with FEV1/FVC.
Conclusion: We demonstrate that CS triggers the modification of exosome components and identify miR-21 derived from bronchial epithelial cells as a mediator of myofibroblast differentiation through the pVHL/HIF-1α signaling pathway, which has potential value for diagnosis and treatment of COPD.
Keywords: cigarette smoking, COPD, airway remodeling, exosome, microRNA
 Global reach, higher impact
Global reach, higher impact