13.3
Impact Factor
Theranostics 2018; 8(21):6008-6024. doi:10.7150/thno.24365 This issue Cite
Research Paper
Nanoparticles targeting extra domain B of fibronectin-specific to the atherosclerotic lesion types III, IV, and V-enhance plaque detection and cargo delivery
1. Center for Nanomedicine and Department of Anesthesiology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02115, USA
2. Department of Pathology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02115
3. Department of Morphology, School of Medicine, Federal University of Ceará, Fortaleza, CE, Brazil
4. KAIST Institute for the BioCentury, Department of Biological Sciences, Korea Advanced Institute of Science and Technology (KAIST), 291 Daehak-ro, Daejeon 34141, Republic of Korea
5. School of Medicine, Federal University of Ceará, Sobral, CE, Brazil
6. Department of Cardiovascular Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02115
7. King Abdulaziz University, Jeddah 21589, Saudi Arabia
Abstract
Extra domain B of fibronectin (FN-EDB) is upregulated in the extracellular matrix during tissue remodeling and has been postulated as a potential biomarker for atherosclerosis, yet no systematic test for FN-EDB in plaques has been reported. We hypothesized that FN-EDB expression would intensify in advanced plaques. Furthermore, engineering of FN-EDB-targeted nanoparticles (NPs) could enable imaging/diagnosis and local delivery of payloads to plaques.
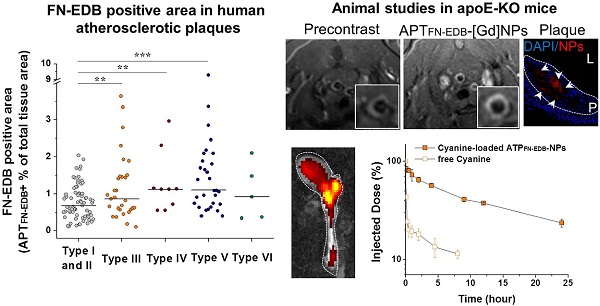
Methods: The amount of FN-EDB in human atherosclerotic and normal arteries (ages: 40 to 85 years) was assessed by histological staining and quantification using an FN-EDB-specific aptide (APTFN-EDB). FN-EDB-specific NPs that could serve as MRI beacons were constructed by immobilizing APTFN-EDB on the NP surface containing DTPA[Gd]. MRI visualized APTFN-EDB-[Gd]NPs administered to atherosclerotic apolipoprotein E-deficient mice in the brachiocephalic arteries. Analysis of the ascending-to-descending thoracic aortas and the aortic roots of the mice permitted quantitation of Gd, FN-EDB, and APTFN-EDB-[Gd]NPs. Cyanine, a model small molecule drug, was used to study the biodistribution and pharmacokinetics of APTFN-EDB-NPs to evaluate their utility for drug delivery.
Results: Atherosclerotic tissues had significantly greater FN-EDB-positive areas than normal arteries (P < 0.001). This signal pertained particularly to Type III (P < 0.01), IV (P < 0.01), and V lesions (P < 0.001) rather than Type I and II lesions (AHA classification). FN-EDB expression was positively correlated with macrophage accumulation and neoangiogenesis. Quantitative analysis of T1-weighted images of atherosclerotic mice revealed substantial APTFN-EDB-[Gd]NPs accumulation in plaques compared to control NPs, conventional MRI contrast agent (Gd-DTPA) or accumulation in wild-type C57BL/6J mice. Additionally, the APTFN-EDB-NPs significantly prolonged the blood-circulation time (t1/2: ~ 6 h) of a model drug and increased its accumulation in plaques (6.9-fold higher accumulation vs. free drug).
Conclusions: Our findings demonstrate augmented FN-EDB expression in Type III, IV, and V atheromata and that APTFN-EDB-NPs could serve as a platform for identifying and/or delivering agents locally to a subset of atherosclerotic plaques.
Keywords: atherosclerosis, aptides, extra domain B of fibronectin, magnetic resonance imaging, nanoparticles
 Global reach, higher impact
Global reach, higher impact