13.3
Impact Factor
Theranostics 2018; 8(22):6274-6290. doi:10.7150/thno.29580 This issue Cite
Research Paper
P-glycoprotein-targeted photodynamic therapy boosts cancer nanomedicine by priming tumor microenvironment
1. Department of Cancer Biology and Comprehensive Cancer Center, Wake Forest University School of Medicine, Winston-Salem, NC 27157, USA;
2. Brain Tumor Center of Excellence, Thomas K Hearn Brain Tumor Research Center, Winston-Salem, NC 27157, USA;
3. Department of Biomedical Engineering, Wake Forest University School of Medicine, Winston-Salem, NC 27157, USA.
Abstract
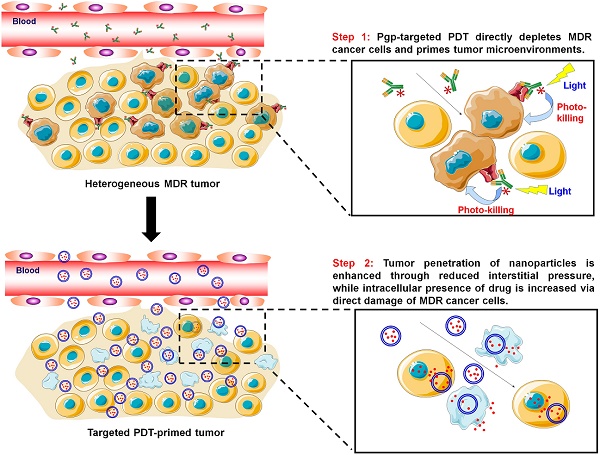
Cancer nanomedicines only modestly improve the overall survival of patients because their anticancer activity is limited by biological barriers posed by the tumor microenvironment. Currently, all the drugs in FDA-approved cancer nanomedicines are substrates for P-glycoprotein (Pgp), and thus, Pgp-mediated multidrug resistance (MDR) remains a hurdle for cancer nanomedicines.
Methods: In this study, Pgp-targeted photodynamic therapy (PDT) was developed to enhance the anticancer efficacy of nanomedicines by depleting MDR cancer cells as well as enhancing tumor penetration of nanomedicines. We first examined the Pgp specificity of our targeted PDT approach, and then tested combination therapy of PDT with Doxil in mixed tumor models of MDR cancer cells and stromal cells, mimicking human heterogeneous tumors.
Results: In vitro studies showed that the antibody-photosensitizer conjugates produced Pgp-specific cytotoxicity towards MDR cancer cells upon irradiation with a near-infrared light. The studies with a co-culture model of MDR cancer cells and stromal cells revealed synergistic effects in the combination therapy of PDT with Doxil. Using a mouse model of mixed tumors containing MDR cancer cells and stroma cells, we observed markedly enhanced tumor delivery of Doxil after PDT in vivo. We further examined the effects of the two modalities on individual cell populations and their synergism using an in vivo dual substrate bioluminescence assay. The results indicated that Pgp-targeted PDT specifically depleted MDR cancer cells and further enhanced Doxil's actions on both MDR cancer cells and stromal cells.
Conclusion: We conclude that our targeted PDT approach markedly enhances anticancer actions of nanomedicines by depleting MDR cancer cells and increasing their tumor penetration, and thereby, may provide an effective approach to facilitate translation of cancer nanomedicines.
Keywords: antibody conjugates, cancer multidrug resistance, cancer nanomedicine, cancer targeted therapy, P-glycoprotein, photodynamic therapy.
 Global reach, higher impact
Global reach, higher impact