13.3
Impact Factor
Theranostics 2019; 9(3):829-836. doi:10.7150/thno.29902 This issue Cite
Research Paper
On the Lipophilic Nature of Autoreactive IgE in Chronic Spontaneous Urticaria
Dept. of Dermatology and Allergy, Charité - Universitätsmedizin Berlin, Germany
Received 2018-9-12; Accepted 2018-11-14; Published 2019-1-25
Abstract
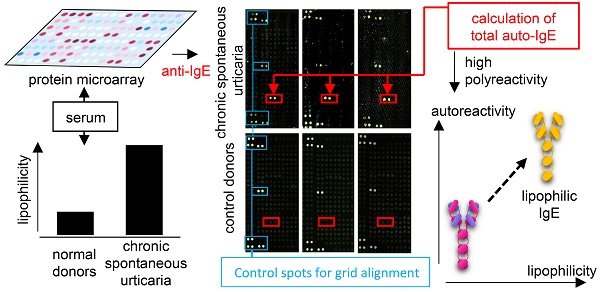
Chronic spontaneous urticaria (CSU) is a skin disease related to autoreactive IgE in at least a subgroup of patients. However, the nature of this autoreactive IgE remains poorly characterized. This investigation had three objectives: first, to quantity CSU autoreactive IgE; second, to recognize the patterns of CSU autoreactive IgE compared with healthy control IgE; and third, to investigate the physiochemical nature of CSU autoreactive IgE.
Methods: IgE autoreactivity was assessed in sera from 7 CSU and 7 healthy individuals. Autoantigen recognition patterns were assessed using principal component analysis (PCA) and heatmap visualization. Lipophilicity was assessed using NanoOrange reagent.
Results: First, although total IgE levels did not differ significantly, the autoreactive proportion of IgE of CSU patients was 62% ± 37%, 1000-fold higher than that of healthy controls 0.03% ± 0.008% (P = 0.0006). Second, CSU autoreactive IgE differed from healthy control IgE by recognizing more and different autoantigens (226 vs. 34; P = 0.01). Third, the median (with 10-90% percentiles) serum level of lipophilic IgE was 39% (38-40%) in 232 CSU patients, 1.4-fold higher than the 28% (26-29%) of 173 healthy controls (P < 0.0001). Furthermore, lipophilicity correlated with autoreactivity (r = 0.8; P < 0.0001), connecting these two observed features.
Conclusion: We believe that these novel observations about CSU autoreactive IgE, particularly the finding that it is more lipophilic than that of IgE from healthy individuals, will lead to the development of new diagnostic tests and therapies for autoreactive IgE-mediated diseases.
Keywords: chronic spontaneous urticaria, IgE, autoreactivity, lipophilicity
Introduction
Chronic spontaneous urticaria (CSU) is an itching, mast cell-driven skin disease characterized by the recurrence of transient wheals, angioedema, or both for more than six weeks [1]. Although CSU is a multifactorial condition involving autoimmunity, coagulation and inflammation [2], IgE-mediated autoimmunity, or autoallergy, is thought to play a major role [3, 4]. This is supported by the following evidences: (i) clinical effectiveness of omalizumab (anti-IgE) in relieving the symptoms of CSU; (ii) scientific observations that CSU patients have higher levels of total IgE than healthy individuals; and (iii) elevated levels of IgE are directed, for example, to thyroid antigens and double-stranded DNA [5, 6]. The last mentioned of these observations has been extended recently by the finding in CSU patients, using protein microarray analysis, of over 200 IgE-autoantigens that are not recognized by healthy controls and that IL-24 is a common, specific, and functional autoantigen of IgE antibodies in patients with CSU [7].
Considering the stronger and broader response to autoallergens in CSU patients as compared with healthy controls, we postulate that CSU is, in at least a subset of patients, an autoallergic disease in which most of the IgE could be of an autoreactive nature. This is of clinical relevance as autoreactive IgE correlates with severity of disease [4] and results in therapeutic consequences [8]. A closer understanding of autoreactive IgE in CSU will pave the way for the development of new diagnostic tests and therapies that will increase the effectiveness of management of the disease.
While the amino acid composition and the resulting physicochemical properties of allergens are quite diverse, cross-reactive, multi-specific IgE has been shown to favour lipophilic ligand-binding interactions [9, 10]. As autoreactivity is often accompanied with cross-reactivity [11, 12], we postulate that the corresponding autoantibody will also be lipophilic.
In this project, we reanalysed a large screening database of autoreactive IgE in CSU patients [7] to calculate total autoreactive IgE levels. Also, we developed an IgE-lipophilicity assay using the fluorescent dye NanoOrange, which binds to lipophilic regions of proteins, resulting in an enhancement of fluorescence [13, 14], to determine lipophilicity of IgE from CSU patients and healthy controls.
Methods
Sera collection
Sera from a total 239 CSU patients (57.1% female) and 180 healthy controls (53.8% female) were used in this study. There were no significant differences between the ages of the CSU patients (median 44, range 15 to 82 years) and healthy controls (median 35, range 20 to 64 years). Seven representative CSU patients (5 female) from the study population and seven age- and sex-matched healthy controls were chosen for auto-IgEome-analysis using microarray screening and IgE-anti-IL-24 measurement. The IgE-lipophilicity assay was performed on the remaining 232 CSU patients and 173 healthy controls. Additional IgE-anti-IL-24 measurements were performed on 27 CSU patients randomly selected from the study population.
CSU patients were recruited from our specialized outpatient clinic for Dermatological Allergology at Charité-Universitätsmedizin Berlin. They had been diagnosed with CSU in line with the current EAACI/GA2LEN/EDF/WAO urticaria guideline [1]. The mean duration of CSU was 5.8 ± 6.5 years and the mean seven-day urticaria activity score [1] was 25 ± 9. All had been treated previously with H1-antihistamines. None had received treatment with anti-IgE or similar agents.
Blood was taken from all study subjects and serum separated after clot formation without the use of a clotting enhancer. After separation, serum was frozen immediately and stored at -80 °C until use.
Blood samples taken from patients and all material investigated in this study were used after written informed consent was obtained, as approved by the ethics committee of the Charité-Universitätsmedizin Berlin (EA1-329-14 and EA1-292-14) according to the Declaration of Helsinki (59th WMA General Assembly, Seoul, October 2008).
Calculation of total autoreactive IgE levels
To assess serum autoallergen levels, we reanalysed the Auto-IgEome data base, which has been published previously [7]. Total IgE levels measured by the Central Laboratory of Charité-Universitätsmedizin Berlin were used to calculate the percentage of autoreactive IgE.
Visualisation of autoantigen recognition patterns
Principal Component Analyses (PCA) and heatmap visualisation were performed using 'ClustVis' [15]. IgE-binding signals to 9374 full-length human proteins as assessed by microarray [7] from seven CSU patients and seven healthy controls were analysed by PCA. Singular value decomposition was used to calculate principal components (PC). The PCA-plot shows PC1 and PC2 indicating 24% and 18.2% of the total variance, respectively. Mean values of the IgE-binding signals for each autoantigen were calculated separately for each sample from both CSU patients and healthy controls. Thirty autoantigens with the highest mean total IgE-binding signal from each group were chosen to generate a heat map and a dendrogram (average linkage). IgE-binding signals were expressed as Z-scores. The rows were labelled with the corresponding GenBank identification number for each autoantigen.
IgE-lipophilicity Assay
Sixteen micrograms of anti-IgE (MHE-18, Biolegend, San Diego, USA) was dispensed in 10 mL Coating Buffer (0.1 M carbonate at pH 9, Merck, Darmstadt, Germany) and 25 μL of this solution was used to coat each well of a black NUNC Maxisorb 384-well plate (Thermofisher, Waltham, USA) at 4 °C overnight. After blocking with 2% human albumin (clinical grade, Baxter, Unterschleißheim, Germany) in TPBS (PBS with 0.05% Tween-20, Merck) overnight at 4 °C, 25 μL human serum was added per well with four replicates and incubated 1 h at room temperature followed by three washes with TPBS and two washes with water. NanoOrange (Thermofisher) dye was processed according to the product instructions (NanoOrange Protein Quantitation Kit, Thermofisher). 5 mL of 1× NanoOrange reagent working solution was prepared and 10 μL of this solution was used per well (without previous heat denaturation of the sample) and incubated for 30 min at room temperature in the dark. The plates were measured in a Victor V reader (Perkin Elmer, Waltham, USA) with standard settings and the results are expressed as counts per second. The percentage of the signal from the maximum signal (maximum signal was set to 100%) of each test was calculated to normalize results from different reproductions.
IgE-anti-IL-24 measurements
The results from the IgE-anti-IL-24 ELISA with units calculated as previously described [7] were used for further analyses. The ratios of IgE-anti-IL-24 to total IgE were calculated as relative levels.
Statistical analyses
Normal distribution was tested using D'Agostino-Pearson omnibus test and an approximately Gaussian distribution served as the basis to perform Student's t-tests corrected for multiple testing by the Holm-Sidak method. The null hypothesis of random results was rejected if the P-value was < 0.05. Statistical differences between results that were not normally distributed were calculated using the Mann Whitney U test.
Results
The majority of IgE in CSU patients is autoreactive IgE
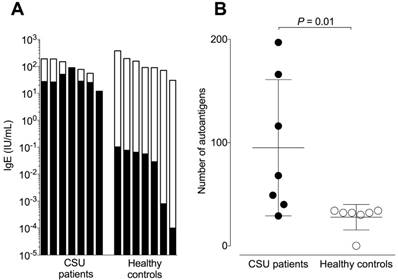
Total IgE levels of the CSU patients and healthy controls did not differ significantly and were 85 ± 61 and 144 ± 115 IU/mL respectively (Figure 1A). The corresponding mean autoreactive IgE levels, calculated by summing the total amount of IgE binding after standardizing the microarray signals with the IgE-anti-IL-24 ELISA, were 37.038 ± 23.916 IU/mL for CSU patients and 0.047 ± 0.039 IU/mL for healthy controls. This difference of ~1000-fold was highly significant (P = 0.001). The absolute amount of autoreactive IgE was comparable in all CSU patients (Figure 1A) and there was no correlation between autoreactive IgE and total IgE (r = 0.16, P = 0.73, data not shown). The relative concentration of autoreactive IgE (expressed as a percentage of total IgE) in CSU patients was 60.96% ± 32.996%, with two patients having 100% of their IgE being autoreactive. In contrast, the relative concentration of autoreactive IgE in healthy controls was only 0.03% ± 0.008%. This two thousand-fold difference was highly significant (P = 0.0006). IgE from CSU patients recognized significantly more autoantigens as compared to healthy controls (total number of 226 vs. 34; P = 0.01; Figure 1B). However, the maximum number of autoantigens detected by IgE in a single CSU patient was 197 compared to 34 in healthy controls.
IgE from CSU patients and healthy controls recognise a different set of autoantigens
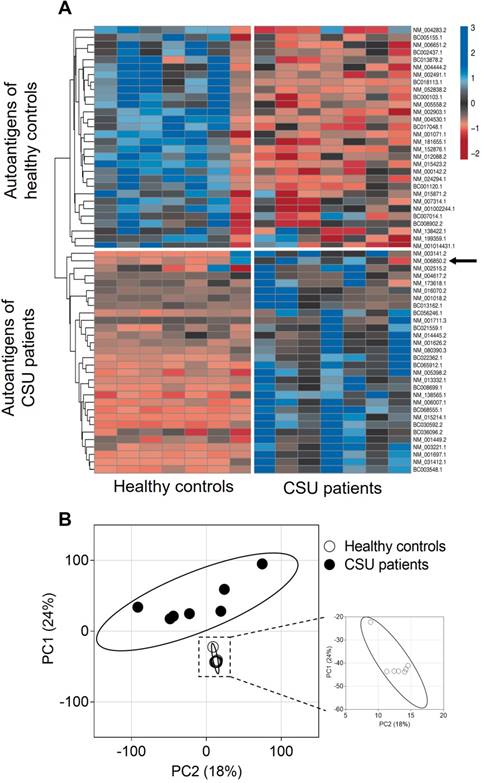
A heat map visualization of the 30 most often recognised, but not necessarily the strongest, autoantigens of IgE from seven CSU patients and the 30 most often recognised autoantigens by IgE from seven healthy controls is shown in Figure 2A. Cluster analysis of these 60 autoantigens results in two different autoantigen groups with little crossover, as indicated by the dendrogram. Visualization of IgE-binding signals to all 9374 full-length human proteins tested using PCA shows again two different autoantigen groups. The CSU patients form a more heterogeneous group, indicated by the wide spread of the data points as compared with the healthy controls (Figure 2B).
CSU patients exhibit high amounts of autoreactive IgE. (A) Logarithmic plot showing total IgE levels (total column length) and autoreactive IgE levels (black areas of columns) of seven CSU patients and seven healthy controls. (B) The mean number of autoantigens recognized by IgE from seven CSU patients (95 ± 66) and seven healthy controls (28 ± 12) were significantly different (P = 0.01, Mann Whitney U test).
Increased lipophilicity is a feature of IgE in CSU patients
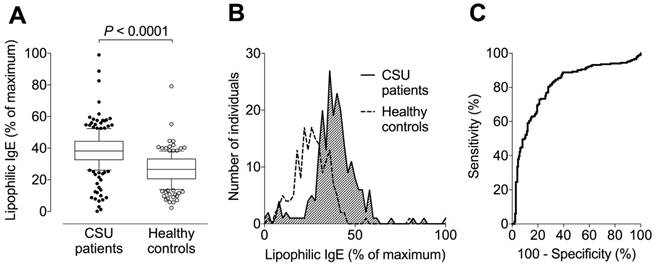
Levels of lipophilic IgE (expressed as a percentage of the maximum signal) were assessed in three independent reproductions. The pooled data with a total of 232 tested CSU patients and 173 healthy controls are shown in Figure 3. The median (with 10-90% percentiles) serum level of lipophilic IgE was 39% (38-40%) in CSU patients, 1.4-fold higher than the 28% (26-29%) in healthy controls (P < 0.0001, Figure 3A-B). ROC-analysis showed a sensitivity of 80% and specificity of 72% (pooled data, Figure 3C) for elevated lipophilicity (cut-off 31% of maximum signal) as determined by use of the Youden Index.
Correlation between autoreactive IgE and IgE-anti-IL-24
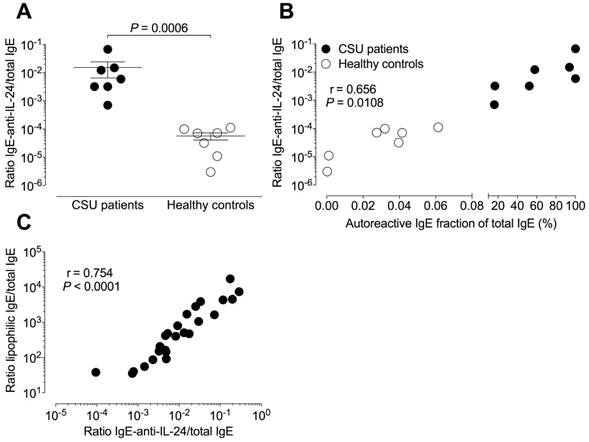
The mean (± SEM) relative IgE-anti-IL-24 level of 15.26×10-3 ± 21.57×10-3 in CSU patients was significantly (P = 0.0006) higher than the 0.06×10-3 ± 0.04×10-3 in healthy controls (Figure 4A). Relative total autoreactive IgE levels (a ratio of summed total microarray binding IgE divided by total IgE) correlated significantly with absolute IgE-anti-IL-24 levels (r = 0.847, P = 0.0001) as well as with relative IgE-anti-IL-24 levels (r = 0.656, P = 0.0108, Figure 4B) in 14 tested individuals (seven CSU patients and seven healthy controls).
IgE-lipophilicity correlates with autoreactive IgE in CSU patients
The ratio of lipophilic IgE to total IgE correlated highly significantly with relative IgE-anti-IL-24 levels (r = 0.754, P < 0.0001, n = 27) in CSU patients (Figure 4C).
IgE from CSU patients detects a different group of autoantigens than IgE from healthy controls. (A) Heat map visualization of the 30 most recognized autoantigens by IgE from seven CSU patients and the 30 most recognized autoantigens by IgE from seven healthy controls. The arrow indicates IL-24 among the CSU autoantigens. Z-scores specify IgE binding to autoantigens measured by microarray. Autoantigens that show similar detection patterns were clustered together, as indicated by the dendrogram on the left. (B) Principal component analyses of IgE-binding signals to 9374 human proteins from seven CSU patients and seven healthy controls. The PCA-plot shows PC1 and PC2 explaining 24% and 18.2% of the total variance, respectively. Prediction ellipses are drawn such that a new observation from the same group will fall inside the ellipse with a probability of 0.95.
Signal intensity to assess lipophilic IgE is significantly higher in CSU patients than healthy controls. (A) Box plots (with 10-90% percentiles) showing that the median serum level of lipophilic IgE in 232 CSU patients is approximately 1.4-fold higher than that of 173 healthy controls. The significance of this difference is P < 0.0001 (Student's t-test). The blot shows pooled data from three independent reproductions with separate populations and similar results. (B) Histogram showing frequency distributions of lipophilic IgE signals (pooled data) from patients with CSU and healthy controls. (C) Receiver operating characteristic curve of three reproductions and pooled data from all measurements.
Relative autoreactive IgE is associated with higher relative IgE-anti-IL-24 levels. IgE is more lipophilic in sera with higher amounts of autoreactive IgE. (A) The mean relative IgE-anti-IL-24 levels (ratio of IgE-anti-IL-24 to total IgE levels) are two log-scales higher in seven CSU patients than in seven healthy controls. The significance of this difference is P = 0.0006 (Mann Whitney U-test). (B) Percentage of autoreactive IgE (from total IgE) correlates with relative IgE-anti-IL-24 levels (N = 14; Pearson correlation coefficient, r = 0.656; P = 0.0108) in combined data from patients with CSU (black circles, N = 7) and healthy controls (open circles, N = 7). (C) The relative level of IgE-anti-IL-24 was used as a surrogate marker for the whole autoreactive IgE concentration. There is a strong correlation between the ratio of lipophilic IgE to total IgE and the ratio of IgE-anti-IL-24 to total IgE in CSU patients (N = 27; Pearson correlation coefficient, r = 0.754; P < 0.0001).
Discussion
This study on the nature of autoreactive IgE in CSU had three major findings: First, the majority of IgE in CSU patients is autoallergic IgE. Second, the IgE of CSU patients recognizes a completely different set of autoantigens than the IgE of healthy controls. Third, the IgE of CSU patients is more lipophilic than that of healthy controls.
The list of diseases in which IgE autoantibodies have been identified is growing and includes atopic dermatitis, pemphigus vulgaris, systemic lupus erythematosus and bullous pemphigoid [4]. Determination of autoreactive IgE levels is of great interest as there is a significant correlation with disease activity [16-19], and autoreactive IgE has implications for therapeutic decision making, for example whether to use omalizumab (anti-IgE) in bullous pemphigoid [8]. In CSU, the prevalence of autoreactive IgE to tissue factor and thyroglobulin is linked to disease activity and is decreased after disease remission following omalizumab treatment [20]. Calculation of the concentration of autoreactive IgE in the sera of CSU patients revealed that it comprised a mean of over 60% of total IgE compared with 0.03% in sera from healthy controls. Similar high amounts of reactive IgE have been reported previously in patients allergic to hydrophobic cyclohexenyl drugs such as propofol [21]. These striking results show the impact of IgE autoantibodies in CSU and the importance of understanding this immune dysregulation.
The results show that the absolute amounts of autoreactive IgE were similar in all CSU patients and independent from their total IgE levels. This is comparable to previous studies demonstrating independence of drug-reactive IgE from total IgE levels [21]. There are several possible explanations for the high, but relatively consistent, levels of autoreactive IgE in CSU. First, IgE production by B cells is controlled in general by an inhibitory feedback mechanism mediated by binding of IgE to CD23 [22]. Second, as autoreactive antibodies are often produced as natural antibodies by B1 B cells, the homeostatic control of those cells might set a maximum amount of autoreactive IgE that can be produced [23, 24].
The large difference between autoreactivity of IgE from CSU patients and healthy controls is an outstanding feature compared with autoreactive IgG patterns [25-30]. However, allergic IgE shows a similar pattern [31]. Full sets of antigens are recognized by IgE from certain individuals, suggesting polyreactive IgE [32]. As IgE, and especially autoreactive IgE, is polyreactive [33, 34], it is possible that one or a few IgE clones are present in healthy controls, which are reactive against the set of autoantigens, or that there are many auto-IgE clones, each one being specific for one or a few autoantigens. However, as almost all of this “normal” autoreactive IgE is not present in CSU patients anymore and a completely new pattern of reactivity is visible, it is more likely that there is only a very limited number of autoreactive IgE clones present, which are poly-autoreactive and which change or are removed as CSU ensues. It is less likely that suddenly all of many normal auto-IgE clones are nearly completely gone in CSU and replaced by a full new set of many CSU auto-IgE clones.
Another major finding in this study is the increased lipophilicity of IgE in CSU patients compared to IgE of healthy controls. Lipophilic IgE has been previously found in patients allergic to lipophilic drugs and in patients suffering from AD but not in healthy controls [21]. Lipophilicity of IgE in these patients was explained by lipophilic antigen epitopes that result in cross reactivity to other lipophilic drugs due to similarities with epitopes binding specific IgE [21]. Cross-reactivity was also observed between the two lipophilic allergens fel d7 and can f1 [35], although it is questioned whether general lipophilic stickiness is alone responsible for cross-reactivity of IgE [36]. However, increased lipophilicity of IgE in CSU patients and the resulting cross-reactivity might explain the association of CSU with hypersensitivity to mugwort and other aeroallergens [37], Staphylococcus aureus enterotoxins [38] and hydrophobic drugs like penicillin [39] leading to increased risk of anaphylactic reactions.
For further analyses of these two observed features, we used IgE-anti-IL-24 as a surrogate marker for autoreactive IgE. Instead of absolute IgE levels, we used relative total IgE levels as they better reflect the physiological conditions and distribution of IgE bound to mast cells [40]. IgE lipophilicity correlates highly significantly with this surrogate marker, suggesting a connection between the autoreactivity and lipophilicity of IgE in CSU patients. This may be a future parameter for general autoreactivity of IgE. As mentioned above, increased lipophilicity of IgE was also found in patients with atopic dermatitis [21], and this disease is known to be associated with IgE autoantibodies against more than 140 autoantigens [41]. Other studies found a tendency for lipophilic immunoglobulin heavy chain complementary-determining regions 3 loops to cause autoreactivity [12, 42, 43]. As the specificity of IgE in patients allergic to grass pollen is mainly determined by the heavy chains [34, 44], the autoreactivity might be due to a preferred heavy chain that is lipophilic. A similar phenomenon was observed for anti-nuclear antibodies as an example of an autoantibody. Although they can derive from almost every heavy chain variable domain gene family, most of them utilize one out of just four different preferred heavy chain variable domain families [45].
In conclusion, in this study we have shown that, unlike in healthy individuals, the majority of IgE in CSU patients is autoallergic IgE and that this IgE recognises a different spectrum of autoantigens than that of IgE from healthy individuals. Furthermore, we have shown that the autoallergic IgE of CSU patients is more lipophilic than that of healthy individuals. We believe that this last finding in particular will pave the way for the development of new diagnostic tests and therapies that will increase the effectiveness of management of CSU.
Abbreviations
auto-IgE: autoreactive IgE; CSU: chronic spontaneous urticaria; IgE-anti-IL-24: IgE reactive to IL-24; IL-24: interleukin-24; PC: principal component; PCA: principal component analyses; ROC: receiver operating characteristic; TPBS: PBS with 0.05% Tween-20.
Acknowledgements
We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité - Universitätsmedizin Berlin. We also thank the Mast Cell-driven Diseases and their Differential Diagnoses (MAD3) team of the Dermatological Allergy (DEAL) group at Charité for help and support. This project was funded by departmental funds.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B. et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. The 2017 revision and update. Allergy. 2018;73:1393-414
2. Asero R, Tedeschi A, Marzano AV, Cugno M. Chronic urticaria: a focus on pathogenesis. F1000Res. 2017;6:1095
3. Kolkhir P, Church MK, Weller K, Metz M, Schmetzer O, Maurer M. Autoimmune chronic spontaneous urticaria: What we know and what we do not know. J Allergy Clin Immunol. 2017;139:1772-81.e1
4. Maurer M, Altrichter S, Schmetzer O, Scheffel J, Church MK, Metz M. Immunoglobulin E-mediated autoimmunity. Front Immunol. 2018;9:689
5. Altrichter S, Peter HJ, Pisarevskaja D, Metz M, Martus P, Maurer M. IgE mediated autoallergy against thyroid peroxidase—a novel pathomechanism of chronic spontaneous urticaria? PLoS One. 2011;6:e14794
6. Hatada Y, Kashiwakura J, Hayama K, Fujisawa D, Sasaki-Sakamoto T, Terui T. et al. Significantly high levels of anti-dsDNA immunoglobulin E in sera and the ability of dsDNA to induce the degranulation of basophils from chronic urticaria patients. Int Arch Allergy Immunol. 2013;161(Suppl 2):154-8
7. Schmetzer O, Lakin E, Topal FA, Preusse P, Freier D, Church MK. et al. IL-24 is a common and specific autoantigen of IgE in chronic spontaneous urticaria. J Allergy Clin Immunol. 2017;142:876-82
8. Sanjuan MA, Sagar D, Kolbeck R. Role of IgE in autoimmunity. J Allergy Clin Immunol. 2016;137:1651-61
9. Dall'antonia F, Pavkov-Keller T, Zangger K, Keller W. Structure of allergens and structure based epitope predictions. Methods. 2014;66:3-21
10. Droupadi PR, Varga JM, Linthicum DS. Mechanism of allergenic cross-reactions—IV. Evidence for participation of aromatic residues in the ligand binding site of two multi-specific IgE monoclonal antibodies. Mol Immunol. 1994;31:537-48
11. Crouzier R, Martin T, Pasquali JL. Heavy chain variable region, light chain variable region, and heavy chain CDR3 influences on the mono- and polyreactivity and on the affinity of human monoclonal rheumatoid factors. J Immunol. 1995;154:4526-35
12. Edwards MR, Brouwer W, Choi CH, Ruhno J, Ward RL, Collins AM. Analysis of IgE antibodies from a patient with atopic dermatitis: biased V gene usage and evidence for polyreactive IgE heavy chain complementarity-determining region 3. J Immunol. 2002;168:6305-13
13. Grossart HP, Steward GF, Martinez J, Azam F. A simple, rapid method for demonstrating bacterial flagella. Appl Environ Microbiol. 2000;66:3632-6
14. Liu Y, Foote RS, Jacobson SC, Ramsey RS, Ramsey JM. Electrophoretic separation of proteins on a microchip with noncovalent, postcolumn labeling. Anal Chem. 2000;72:4608-13
15. Metsalu T, Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res. 2015;43:W566-70
16. Schmid-Grendelmeier P, Fluckiger S, Disch R, Trautmann A, Wuthrich B, Blaser K. et al. IgE-mediated and T cell-mediated autoimmunity against manganese superoxide dismutase in atopic dermatitis. J Allergy Clin Immunol. 2005;115:1068-75
17. Nagel A, Lang A, Engel D, Podstawa E, Hunzelmann N, de Pita O. et al. Clinical activity of pemphigus vulgaris relates to IgE autoantibodies against desmoglein 3. Clin Immunol. 2010;134:320-30
18. Bayry J. Lupus pathogenesis: role of IgE autoantibodies. Cell Res. 2016;26:271-2
19. Dema B, Pellefigues C, Hasni S, Gault N, Jiang C, Ricks TK. et al. Autoreactive IgE is prevalent in systemic lupus erythematosus and is associated with increased disease activity and nephritis. PLoS One. 2014;9:e90424
20. Cugno M, Asero R, Ferrucci S, Lorini M, Carbonelli V, Tedeschi A. et al. Elevated IgE to tissue factor and thyroglobulin are abated by omalizumab in chronic spontaneous urticaria. Allergy. 2018 [Epup ahead of print]
21. Gueant JL, Mata E, Masson C, Gerard P, Moneret-Vautrin DA, Mouton-Faivre C. et al. Non-specific cross-reactivity of hydrophobic serum IgE to hydrophobic drugs. Mol Immunol. 1995;32:259-66
22. Fellmann M, Buschor P, Rothlisberger S, Zellweger F, Vogel M. High affinity targeting of CD23 inhibits IgE synthesis in human B cells. Immun Inflamm Dis. 2015;3:339-49
23. Macias-Garcia A, Heizmann B, Sellars M, Marchal P, Dali H, Pasquali JL. et al. Ikaros is a negative regulator of B1 cell development and function. J Biol Chem. 2016;291:9073-86
24. Durand CA, Hartvigsen K, Fogelstrand L, Kim S, Iritani S, Vanhaesebroeck B. et al. Phosphoinositide 3-kinase p110 delta regulates natural antibody production, marginal zone and B-1 B cell function, and autoantibody responses. J Immunol. 2009;183:5673-84
25. Haddon DJ, Diep VK, Price JV, Limb C, Utz PJ, Balboni I. Autoantigen microarrays reveal autoantibodies associated with proliferative nephritis and active disease in pediatric systemic lupus erythematosus. Arthritis Res Ther. 2015;17:162
26. Chong BF, Tseng LC, Lee T, Vasquez R, Li QZ, Zhang S. et al. IgG and IgM autoantibody differences in discoid and systemic lupus patients. J Invest Dermatol. 2012;132:2770-9
27. Rinaldi S, Brennan KM, Kalna G, Walgaard C, van Doorn P, Jacobs BC. et al. Antibodies to heteromeric glycolipid complexes in Guillain-Barre syndrome. PLoS One. 2013;8:e82337
28. Bruserud O, Oftedal BE, Landegren N, Erichsen MM, Bratland E, Lima K. et al. A longitudinal follow-up of autoimmune polyendocrine syndrome type 1. J Clin Endocrinol Metab. 2016;101:2975-83
29. Halstead SK, Kalna G, Islam MB, Jahan I, Mohammad QD, Jacobs BC. et al. Microarray screening of Guillain-Barre syndrome sera for antibodies to glycolipid complexes. Neurol Neuroimmunol Neuroinflamm. 2016;3:e284
30. Galban-Horcajo F, Vlam L, Delmont E, Halstead SK, van den Berg L, van der Pol WL. et al. The diagnostic utility of determining anti-GM1: GalC complex antibodies in multifocal motor neuropathy: a validation study. J Neuromuscul Dis. 2015;2:157-65
31. Simpson A, Lazic N, Belgrave DC, Johnson P, Bishop C, Mills C. et al. Patterns of IgE responses to multiple allergen components and clinical symptoms at age 11 years. J Allergy Clin Immunol. 2015;136:1224-31
32. Saarelainen S, Rytkonen-Nissinen M, Rouvinen J, Taivainen A, Auriola S, Kauppinen A. et al. Animal-derived lipocalin allergens exhibit immunoglobulin E cross-reactivity. Clin Exp Allergy. 2008;38:374-81
33. Zeller S, Glaser AG, Vilhelmsson M, Rhyner C, Crameri R. Immunoglobulin-E-mediated reactivity to self antigens: a controversial issue. Int Arch Allergy Immunol. 2008;145:87-93
34. Collins AM, Sewell WA, Edwards MR. Immunoglobulin gene rearrangement, repertoire diversity, and the allergic response. Pharmacol Ther. 2003;100:157-70
35. Apostolovic D, Sanchez-Vidaurre S, Waden K, Curin M, Grundstrom J, Gafvelin G. et al. The cat lipocalin Fel d 7 and its cross-reactivity with the dog lipocalin Can f 1. Allergy. 2016;71:1490-5
36. James LC, Tawfik DS. The specificity of cross-reactivity: promiscuous antibody binding involves specific hydrogen bonds rather than nonspecific hydrophobic stickiness. Protein Sci. 2003;12:2183-93
37. de Vos G, Kravvariti E, Collins J, Tavdy A, Nazari R, Hudes G. et al. Increased allergic sensitization to mugwort in chronic urticaria. Dermatology. 2012;225:141-6
38. Altrichter S, Hawro T, Liedtke M, Holtappels G, Bachert C, Skov PS. et al. In chronic spontaneous urticaria, IgE against staphylococcal enterotoxins is common and functional. Allergy. 2018;73:1497-504
39. Silverman S, Localio R, Apter AJ. Association between chronic urticaria and self-reported penicillin allergy. Ann Allergy Asthma Immunol. 2016;116:317-20
40. Vultaggio A, Virgili G, Gaeta F, Romano A, Maggi E, Matucci A. High serum beta-lactams specific/total IgE ratio is associated with immediate reactions to beta-lactams antibiotics. PLoS One. 2015;10:e0121857
41. Cipriani F, Ricci G, Leoni MC, Capra L, Baviera G, Longo G. et al. Autoimmunity in atopic dermatitis: biomarker or simply epiphenomenon? J Dermatol. 2014;41:569-76
42. Lipsanen V, Walter B, Emara M, Siminovitch K, Lam J, Kaushik A. Restricted CDR3 length of the heavy chain is characteristic of six randomly isolated disease-associated VH J558+ IgM autoantibodies in lupus prone motheaten mice. Int Immunol. 1997;9:655-64
43. Larimore K, McCormick MW, Robins HS, Greenberg PD. Shaping of human germline IgH repertoires revealed by deep sequencing. J Immunol. 2012;189:3221-30
44. Gadermaier E, Flicker S, Lupinek C, Steinberger P, Valenta R. Determination of allergen specificity by heavy chains in grass pollen allergen-specific IgE antibodies. J Allergy Clin Immunol. 2013;131:1185-93 93 e1-6
45. Chen L, Chang S, Mohan C. Molecular signatures of antinuclear antibodies-contributions of heavy chain CDR residues. Mol Immunol. 2002;39:333-47
Author contact
![]() Corresponding author: Prof. Dr. Marcus Maurer, Charité - Universitätsmedizin Berlin. Dept. of Dermatology and Allergy, Chariteplatz 1, 10117 Berlin, Germany. E-Mail: marcus.maurerde; Tel. +49 (0)30 450 518 043; Fax. +49 (0)30 450 518 972
Corresponding author: Prof. Dr. Marcus Maurer, Charité - Universitätsmedizin Berlin. Dept. of Dermatology and Allergy, Chariteplatz 1, 10117 Berlin, Germany. E-Mail: marcus.maurerde; Tel. +49 (0)30 450 518 043; Fax. +49 (0)30 450 518 972
 Global reach, higher impact
Global reach, higher impact