13.3
Impact Factor
Theranostics 2019; 9(6):1728-1740. doi:10.7150/thno.30915 This issue Cite
Research Paper
MMP-2-Controlled Transforming Micelles for Heterogeneic Targeting and Programmable Cancer Therapy
1. Center for Neuroscience Research, School of Basic Medical Sciences, Fujian Medical University, Fuzhou, Fujian Province, 350108, China.
2. CAS Key Laboratory of Standardization and Measurement for Nanotechnology, CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology of China, Beijing 100190, China;
3. Sino-Danish College, University of Chinese Academy of Sciences, Beijing 100049, P. R. China;
4. Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China;
5. Department of Diagnostic Imaging National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College
6. School of Life Science, Beijing Institute of Technology, Beijing, 100081, China
Abstract
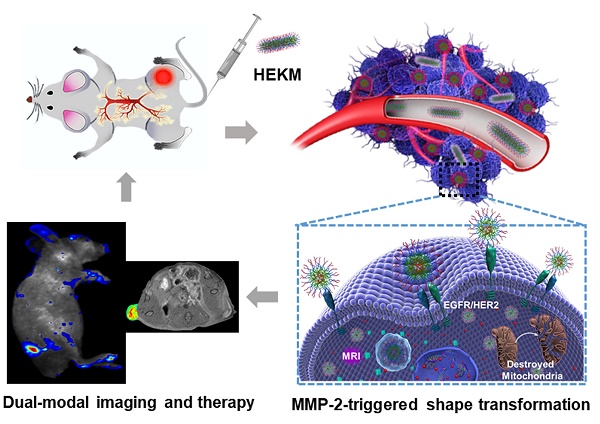
Herein, through the active-peptide-functionalization, we developed a nanoscale micelles system (named HEKM) which consists of tumor microenvironment-regulated shape-changing with specific recognition abilities for enhanced cellular targeting, internalization and therapy of heterogeneic tumors. As a result, HEKMs could recognize and bind the tumor heterogeneity marker EGFR-HER2 complex, which led to an enhanced tumor targeting effect. In particular, HEKMs could self-assemble into nanorods under normal physiological conditions while transform into nanospheres in the tumor extracellular microenvironment by a sensitive response to matrix metalloproteinase-2 (MMP-2). The nanorods could prolong the blood circulation time while the nanospheres could accelerate tissue penetration in tumors. In vivo dual-modal targeted imaging was realized by FRET-fluorophore conjugation and gadolinium loading in HEKMs. Tumor cell apoptosis was achieved by proapoptotic element integration. The in vitro and in vivo studies both demonstrated that these rationally designed, shape-changing and targeting micelles could achieve maximized drug efficacy and minimum side effects.
Keywords: specific targeting peptide, MMP-2-controlled, shape transformation, dual-modal imaging, cancer therapy
 Global reach, higher impact
Global reach, higher impact